Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Author: Hon A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand, 2011. Updated by Nigel Maher, Anatomic Pathology Registrar, Sydney, Australia. December 2017.
Introduction
Demographics
Clinical features
Differential diagnoses
Causes
Tests
Breslow thickness
Clark level of invasion
Treatment
Staging
Lymph node removal
Follow-up
Outlook
Desmoplastic melanoma is a rare form of invasive melanoma, a skin cancer that arises from pigment cells (melanocytes).
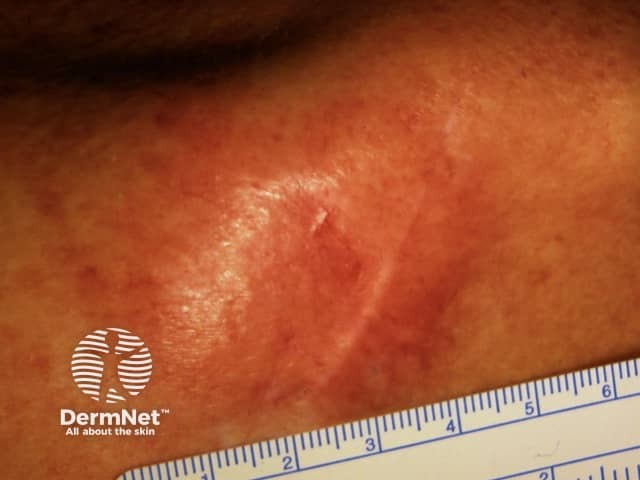
Desmoplastic melanoma
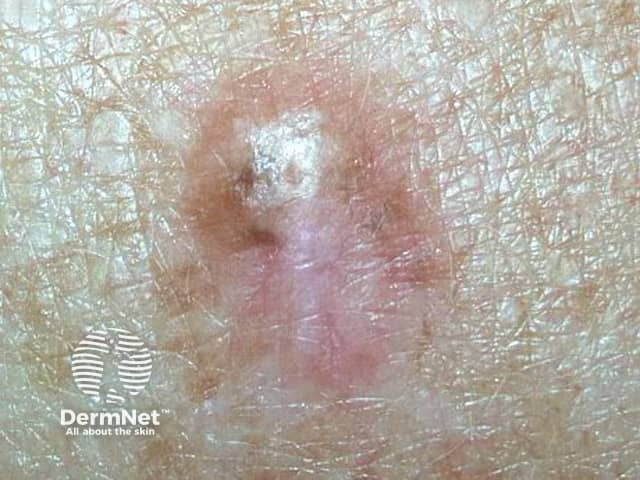
Image supplied by MoleMap NZ
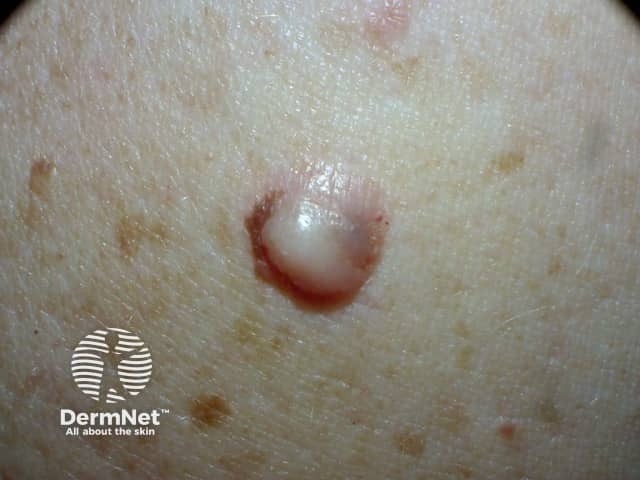
Image supplied by MoleMap NZ
Management of melanoma is evolving. For up to date recommendations, refer to Australian Cancer Council Clinical practice guidelines for the diagnosis and management of melanoma
Desmoplastic melanoma accounts for about 1% of melanoma in Australia and New Zealand.
The main risk factors for desmoplastic melanoma are:
Desmoplastic melanoma is most common on sun-exposed areas of the head and neck (> 50%). Desmoplastic melanoma usually lacks the ABCD melanoma warning signs: Asymmetry, Border irregularity, Colour variation, large Diameter.
It presents as a slowly enlarging area of thickened skin, sometimes described as scar-like. It is often skin coloured but may be pigmented. It becomes more distinctive in time, often growing over months to years before it is recognised. Features include:
Deep invasive melanoma often has the following features:
Not uncommonly, desmoplastic melanoma can be mistaken for other benign lesions, such as:
Desmoplastic melanoma is due to the development of malignant pigment cells (melanocytes) within the dermis. These cells may arise within another type of melanoma or in previously normal-appearing skin. What triggers the melanocytes to become malignant is unknown, but it is likely to be a series of changes to DNA.
It is essential to diagnose desmoplastic melanoma accurately. A careful clinical history is important, which includes noting previous treatments received for non-resolving lesions. Palpation is an important step, as a large majority of desmoplastic melanoma are indurated. Clinical diagnosis is aided by dermoscopy and skin biopsy (usually excision biopsy). Depending on the thickness and proportion of desmoplasia within the invasive melanoma, sentinel lymph node biopsy, imaging studies and blood tests may be advised.
As desmoplastic melanoma is commonly found on the head and neck, and with lentigo maligna, it is possible that if such lesions are only partially biopsied, the desmoplastic melanoma may be missed. Reflectance confocal microscopy may be useful to help guide the site of the biopsy.
Dermoscopy can be helpful in distinguishing desmoplastic melanoma from other skin lesions. The most frequently observed dermatoscopic features of desmoplastic melanoma are:
If the skin lesion is suspicious of desmoplastic melanoma, it should be urgently cut out (excision biopsy).
The pathological diagnosis of melanoma can be very difficult. Histopathological features of desmoplastic melanoma include:
Immunohistochemical stains for melanoma may be helpful.
The pathologist's report should include a macroscopic description of the specimen and melanoma (the naked eye view) and a microscopic description.
The report may also include comments about the cell type and its growth pattern, invasion of blood vessels or nerves, inflammatory response, regression and whether there is an associated naevus or another form of melanoma.
Desmoplastic melanoma is categorised into either ‘pure’ or ‘mixed’ subtypes, which reflect the extent of desmoplasia. The mixed subtypes include a component of spindle cell melanoma.
The Breslow thickness is reported for invasive melanomas. It is measured vertically in millimetres from the top of the granular layer (or base of superficial ulceration) to the deepest point of tumour involvement. In most melanomas, it is a strong predictor of outcome; the thicker the melanoma, the more likely it is to metastasise.
In desmoplastic melanoma, the Breslow thickness is typically thick (3–4 mm). Disease-free survival is worse for melanoma with Breslow thickness > 4 mm, but is slightly more favourable for pure desmoplastic melanoma than other forms of melanoma of the same thickness.
The Clark level indicates the anatomic plane of invasion. The deeper the Clark level, the greater the risk of metastasis (secondary spread). It is not considered useful in predicting outcome in desmoplastic melanoma.
The initial treatment of a desmoplastic melanoma is surgical excision.
Adjuvant radiation therapy may be considered for pure desmoplastic melanoma, Breslow thickness > 4 mm, perineural invasion or positive surgical margins.
Melanoma staging means analysing primary tumour characteristics (including the Breslow thickness and presence of ulceration), the spread of melanoma to the lymph nodes, the spread of melanoma to loco-regional or distant sites, and the lactate dehydrogenase (LDH) level.
Most melanoma specialists refer to the American Joint Committee on Cancer (AJCC) cutaneous melanoma staging guidelines (8th Edition). The same staging criteria are used in all melanomas, irrespective of their growth pattern.
Stage 0 — In-situ melanoma
Stage 1 — Thin melanoma < 2 mm in thickness
Stage 2 — Thick melanoma > 2 mm in thickness, or > 1mm thickness with ulceration
Stage 3 — Melanoma spread to involve local lymph nodes
Stage 4 — Distant metastases have been detected
If the local lymph nodes are enlarged due to metastatic melanoma, they should be completely removed. This requires a surgical procedure, usually under general anaesthetic. If they are not enlarged, they may be tested to see if there is any microscopic spread of melanoma. The test is known as a sentinel node biopsy.
In New Zealand, many surgeons recommend sentinel node biopsy for melanomas thicker than 1 mm, especially in younger persons. However, although the biopsy may help in staging cancer, it does not offer any survival advantage. The necessity for sentinel node biopsy is controversial at present.
Lymph nodes containing metastatic melanoma often increase in size quickly. An involved node is usually non-tender and firm to hard in consistency. If this occurs between planned follow-up visits, let your doctor know promptly.
Desmoplastic melanoma has a high mutational load, which may increase the chance of response to immunotherapy with anti-PD-1/PD-1L medications such as pembrolizumab or nivolumab.
The main purpose of follow-up is to detect recurrences early, but it also offers an opportunity to diagnose a new primary melanoma at the first possible opportunity.
The Australian and New Zealand Guidelines for the Management of Melanoma (2008) make the following recommendations for follow-up for patients with invasive melanoma.
Follow-up appointments may include:
In those with more advanced primary disease, follow-up may include:
Tests are not typically worthwhile for Stage 1 or Stage 2 melanoma patients unless there are signs or symptoms of disease recurrence or metastasis. No routine tests are necessary for healthy patients who have remained well for five years or longer after removal of their melanoma, whatever stage.
The risk of spread and ultimate death from invasive melanoma depends on several factors, but the main one is the measured thickness of the melanoma at the time it was surgically removed. There are conflicting reports about prognostic indicators and outcomes for desmoplastic melanoma, due to its rarity, differences in pathological interpretation, and differing study designs/methodology. The results of the three studies are described below.