Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Authors: Dr Ian Coulsons, Dermatologist and Editor-in-Chief, United Kingdom (2023); Vanessa Ngan, Staff Writer (2003).
Edited by the DermNet content department.
Introduction
What is it and how does it work?
Uses
How to use
Side effects
Photodynamic therapy (PDT) is a treatment used mainly for superficial types of skin cancer. PDT is effective in treating actinic keratoses, in situ squamous cell carcinoma (Bowen disease), and superficial basal cell carcinomas. It may also be used for treatment of small, thin, low-risk nodular basal cell carcinomas outside of the head and neck area.
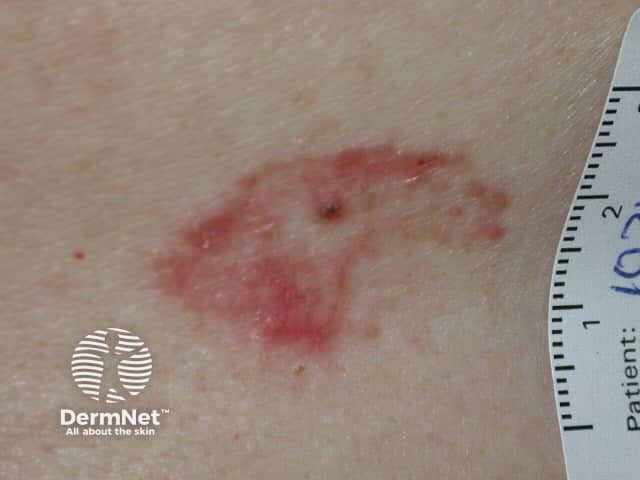
Superficial basal cell carcinoma
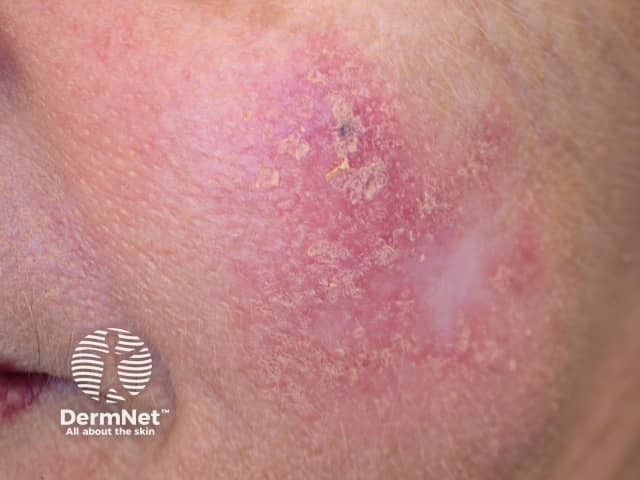
Intraepidermal carcinoma
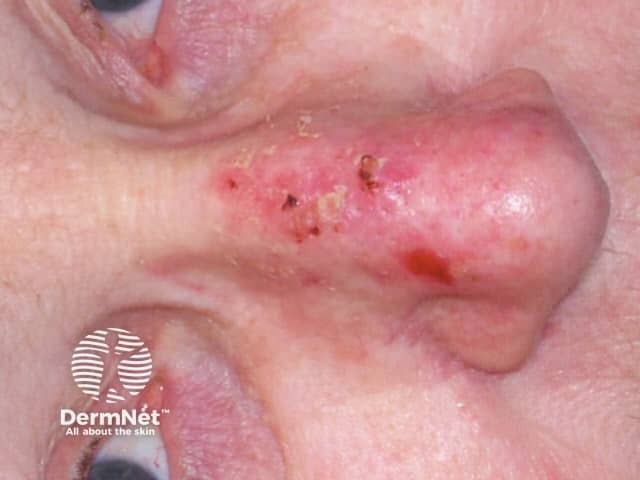
Actinic keratoses
PDT is also sometimes used off-label for facial rejuvenation and to treat mild to moderate acne, and a variety of skin infections including wound infections, onychomycosis, viral warts, and cutaneous leishmaniasis. Several other conditions have also been studied and may respond to PDT such as psoriasis, mycosis fungoides, and extramammary Paget disease.
PDT utilises photosensitising agents, oxygen and light, to create a photochemical reaction that selectively destroys cancer cells. Photosensitising agents are drugs that are administered into the body through topical, oral or intravenous methods. In the body, they concentrate in cancer cells and only become active when light of a certain wavelength is directed onto the area where the cancer is. The photodynamic reaction between the photosensitising agent, light and oxygen produces reactive oxygen species (singlet oxygen, hydroxyl radicals, and superoxides) which interact with cellular structures and cause cell death.
Methyl aminolevulinic acid cream
Aminolevulinic acid hydrochloride topical solution
BF-200 ALA gel
Porfimer sodium
Benzoporphyrin derivative monoacid ring A
Tin ethyl etiopurpurinLutetium texaphyrin
Light sources used in PDT include laser, nonlaser, and ambient daylight.
Laser light has the advantages of being:
Laser light is suitable for small skin lesions whilst nonlaser light is better for the treatment of large skin lesions as the field of illumination is larger.
Nonlaser light that emits polychromatic light is also suitable when using different photosensitisers with different absorption maxima. They are considerably cheaper than laser sources.
Natural daylight is used successfully as a light source for the treatment of actinic keratoses. It has the advantage of being readily available and free, but 2 hours exposure is needed. In some locations and seasons, it is not reliable or of sufficient intensity (see methyl aminolevulinic photodynamic therapy).
Ambulatory devices containing the photosensitiser and a battery operated LED source have been developed for single, small, premalignant, non-melanoma skin cancers.

Irradiation of a lesion on the left cheek with a red light source
PDT is currently being used or investigated as a treatment for the following skin conditions:
Stage 1
Stage 2
Stage 3
Recent evidence suggests that topical PDT may be more effective if the patient is treated with high dose oral vitamin D for 7 days before the treatment is administered.
Side effects from PDT are due to the treated area being sensitive to light. The photosensitivity usually lasts about 24 hours (depending on the specific agent). Side effects may include:
The treated area should be protected from light exposure using a dressing. A local anaesthetic such as lignocaine (lidocaine) spray may be applied to the treatment area before or during Stage 2 of the procedure to help relieve pain.
The treated skin lesion may blister and ulcerate as the cancer cells die off. This may take several weeks to heal. Scarring is generally minimal (but can be moderate). Loss of pigmentation may occur sometimes and can be permanent.
Although photosensitising drugs concentrate in cancer cells, they can also make healthy cells more sensitive to light. This is not a problem when photosensitising creams are used as they are localised to the treatment site. It is more of a problem when photosensitising drugs are given by mouth or injected intravenously. These patients may find all parts of their body sensitive to light and should take precautions to protect themselves from light for the necessary period of time (may be days or weeks depending on the photosensitising drug used).
New Zealand approved datasheets are the official source of information for these prescription medicines, including approved uses and risk information. Check the individual New Zealand datasheet on the Medsafe website.