Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Author: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand, 1998. Updated by Dr Anna Yu Luo, Medical Registrar, Department of General Medicine, Rotorua Hospital, Rotorua, NZ. DermNet Editor in Chief: Adjunct A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand. Copy edited by Gus Mitchell/Maria McGivern. November 2018.
Introduction Demographics Contraindications More information Benefits Disadvantages Side effects and risks
Ciclosporin is an immunosuppressant medication that is used to treat some inflammatory illnesses. It is used for conditions that affect the skin and for conditions that affect other body organs. In New Zealand, ciclosporin is available and fully funded as a tablet and as an oral liquid. It is also available unfunded as eye drops and as an injection [1].
Ciclosporin works by selectively blocking calcineurin, a calcium-dependent serine-threonine protein phosphatase found in the T cells of the immune system. A calcineurin inhibitor reduces T-cell activation and the activity of the immune system [2].

Atopic dermatitis
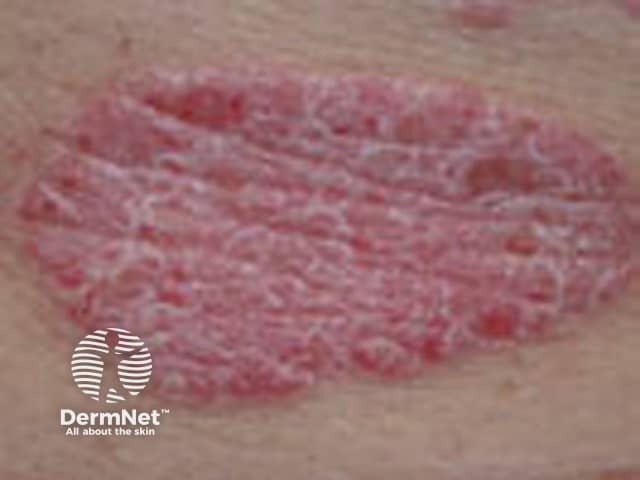
Psoriasis
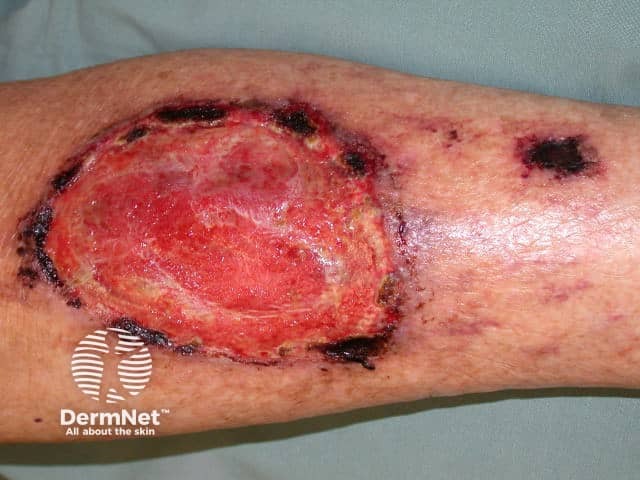
Pyoderma gangrenosum
In dermatology, ciclosporin can be used as a systemic agent for adults and children with severe skin conditions that are not adequately treated with topical therapies alone.
Common skin conditions that ciclosporin is used to treat are atopic eczema/dermatitis and psoriasis.
Less common skin conditions for which ciclosporin is sometimes used off-licence include:
Other systemic uses of ciclosporin include to:
Topical ciclosporin eye drops can be used for keratoconjunctivitis and endogenous uveitis [4]. When used as an eye drop, there are usually undetectable levels of ciclosporin absorbed into the bloodstream [4].
There are other off-label uses of ciclosporin, and cases where ciclosporin has been effective, even though it is not usually used for those conditions. Such examples include the treatment of Stevens–Johnson / toxic epidermal necrolysis, and treatment of severe acute ulcerative colitis that has not responded to other treatments [1,5].
People who should not use ciclosporin include those who have:
In most cases, people who develop these conditions while on ciclosporin should have the ciclosporin withheld or discontinued [1].
Ciclosporin is a small lipophilic (fat-binding) protein, made up of a circular chain of 11 amino acids, which was originally derived from fungi. It is absorbed by the intestine after oral administration and is metabolised by cytochrome P-450 3A enzymes in the liver. Ciclosporin is then excreted in the bile [2,6].
All systemic forms of ciclosporin should initially be administered twice a day (every 12 hours), at the same time each day. Its metabolism differs between adults and children. In children, ciclosporin is likely to be absorbed more slowly and cleared more quickly, so children may need to take ciclosporin three times a day.
When given orally, ciclosporin takes approximately 1–8 hours to reach peak blood concentrations. Its elimination half-life is variable.
There are now two forms of ciclosporin: a modified version and the original, unmodified formulation [6].
Ciclosporin does not have to be given with food but should be given at the same time about meals. The liquid formulation can be mixed with fruit juice (except grapefruit juice) to improve the taste but should be given straight after mixing. Ciclosporin solutions can adhere to plastic, so should be given in a non-plastic container [6].
The administered dose of ciclosporin is based on the patient's body weight and the condition being treated, usually at a dosage of 2–15 mg/kg per day in divided doses. Adjustments to the dose are made based on the improvement of symptoms and adverse effects. Blood tests for ciclosporin concentrations in the blood can also be helpful; these blood tests are done in one of two ways:
The dose can then be increased or decreased by 25–50 mg at the time of the next administration [2,6].
After disease remission is achieved, usually after 6–8 weeks of treatment, the dose of ciclosporin can be slowly reduced. Ciclosporin can eventually be given intermittently, such as twice weekly, as maintenance therapy. This is often continued for a longer period, such as up to 1 year [7,8].
Ciclosporin is an effective medication that can be used for a wide range of conditions.
Ciclosporin is a safe medication when used under the guidance and monitoring of an experienced health professional.
Ciclosporin can interact with some food, such as grapefruit, and other medications. It is important to check for interactions before starting any new medication. Some common medications that interact with ciclosporin include:
These medications should be avoided, or if not possible, ciclosporin levels should be monitored closely [1,2,6]. Given the risk of toxicity to the kidneys and other body organs, regular general blood tests, and monitoring by a doctor is required during treatment with ciclosporin.
Ciclosporin is a category B medication. There are limited studies regarding the use of ciclosporin in pregnancy and breastfeeding, and the safety of this medication is not well-established. Although it has previously been used safely and has demonstrated non-teratogenicity (ie, it does not cause birth defects), it should be avoided unless the benefits outweigh potential harm [1,2,8,9]. There are specific concerns about the risk of inducing hypertension.
There are limited studies on the use of ciclosporin for skin conditions in older people. Caution is needed to avoid drug–drug interactions and the aggravation of any comorbidities, such as poor renal function [8,9].
There is a risk of recurrence or flare of diseases, such as a worsening of atopic eczema or psoriasis, after ciclosporin treatment is discontinued. This is mitigated by reducing the dose slowly and gradually [10].
Ciclosporin has important potential risks. The risk of side effects increases with a longer duration of treatment. Common side effects include:
Other important risks with ciclosporin include:
See also, Cutaneous adverse reactions to calcineurin inhibitors.

Hypertrichosis due to ciclosporin
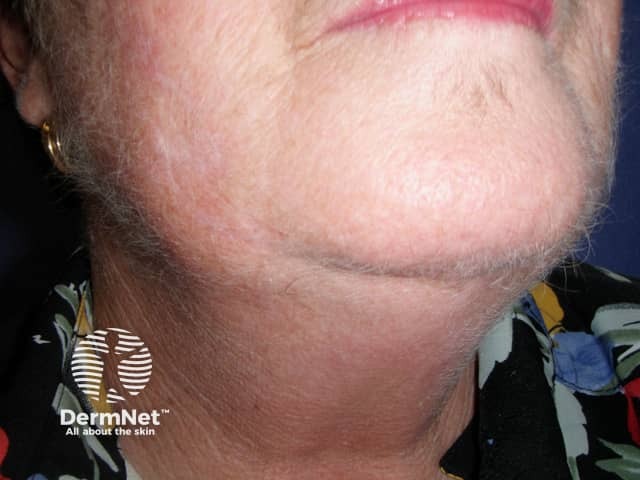
Hypertrichosis due to ciclosporin
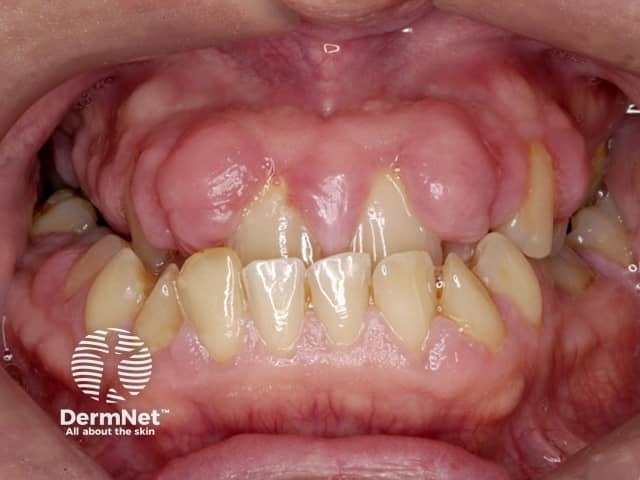
Gum hypertrophy due to ciclosporin
It is important to evaluate each person’s risk before beginning treatment with ciclosporin. A general physical examination should be done before starting ciclosporin.
Blood tests are undertaken for kidney function, liver function, full blood count, fasting lipid profile, electrolytes, and screening for chronic infections.
Routine immunisations may need to be reviewed and updated, such as those for measles and varicella-zoster [3].
Patients can reduce the risk of developing side effects by modifying their lifestyle. Patients on ciclosporin should:
It is recommended that blood pressure is measured once to twice-weekly for the first month of treatment, then monthly after that for the first three months, and then less frequently after that [3].
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).