What is hydroxyurea?
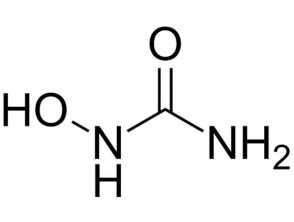
Hydroxyurea (CH4N2O2), also known as hydroxycarbamide, is an enzyme inhibitor used mainly to treat blood disorders. Hydroxyurea is usually administered as an oral capsule or tablet.
Chemical structure of hydroxyurea
Who uses hydroxyurea?
Hydroxyurea is frequently used in the treatment of chronic myeloproliferative diseases such as chronic myelocytic leukaemia, essential thrombocythaemia, and polycythaemia vera. It is also increasingly prescribed for sickle cell anaemia, and to treat refractory ovarian cancer and some head and neck cancers.
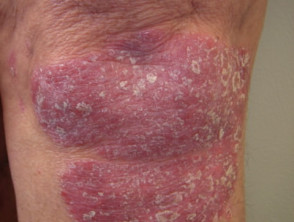
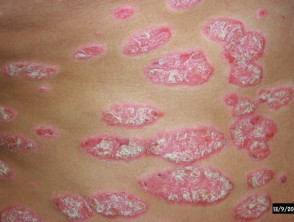
In dermatology, hydroxyurea has a role in treating psoriasis, and hypereosinophilic syndrome, and should be considered for human immunodeficiency virus-associated psoriasis.
Hydroxyurea was found to be an effective systemic treatment for psoriasis in 1970, but is used ‘off-label’. Psoriasis is not an approved treatment for psoriasis, mainly due to the availability of newer and/or more effective systemic treatments.
Hydroxyurea has been used off-label for the treatment of hypereosinophilic syndrome since 1978, usually in combination with systemic corticosteroids.
Hydroxyurea should not be used in pregnancy as it is teratogenic, and women of child-bearing age must be using reliable birth-control measures during hydroxyurea treatment and for at least six months after stopping. Men taking hydroxyurea should also use effective birth-control measures during treatment and for at least 12 months after completing treatment.
Skin conditions can be treated with hydroxyurea
Tell me more about hydroxyurea.
Hydroxyurea was first synthesised in the 1860s, but its use as a cancer therapy was not reported until 1960. Hydroxyurea inactivates the enzyme ribonucleotide reductase, therefore preventing DNA synthesis and repair. It synchronises the cell cycle in the G1 phase, and kills cells in the S phase.
The mechanism of action of hydroxyurea in treating psoriasis is assumed to be the inhibition of skin cell proliferation through its effects on DNA synthesis, and/or by causing gene hypomethylation which induces cell differentiation.
Enzyme inhibition by hydroxyurea also results in anti-retroviral effects, so could be considered in patients with HIV-associated psoriasis.
The oral bioavailability of hydroxyurea is almost 100% and it is distributed across all body tissues. The serum concentration peaks two hours after oral ingestion and has been cleared by 24 hours. Tissue concentration peaks at 8 hours and persists for 20 hours. Hydroxyurea is excreted by the kidneys.
What is hydroxyurea used for?
Psoriasis
- This is an off-label use of hydroxyurea — it does not have FDA or European approval for this indication.
- Improvement in symptoms begins about two weeks after starting oral hydroxyurea, and is maximal after eight weeks.
- Efficacy profile is best seen in plaque psoriasis. Unstable or fulminant psoriasis should be treated with other agents.
- Initial suggested dose 500mg twice daily, maximum dose 2g/d.
- In HIV patients with psoriasis, an initial dose of 500mg/d has been recommended, increasing to 1.5g/d if tolerated.
- Hydroxyurea should be ceased if there has been no response after 8 weeks.
- Gradual weaning from hydroxyurea is advised once a satisfactory clinical response has been achieved. It is not used long-term for psoriasis.
- Hydroxyurea may be cautiously combined with other psoriasis treatments.
- Hydroxyurea is not effective for psoriatic arthritis.
- There is insufficient data to recommend hydroxyurea use in children with psoriasis.
Hypereosinophilic syndrome
- This is an off-label use of hydroxyurea.
- Hydroxyurea is used as a steroid-sparing agent allowing reduction of corticosteroid dose for those experiencing steroid side effects or where the prednisone maintenance dose exceeds 10mg/day.
- Initial dose of 500mg/d, increasing to a maximum of 2g/d as tolerated.
Non-cutaneous squamous cell carcinoma of the head and neck
- Hydroxyurea causes cell cycle G1/S phase arrest which holds cells in a radiosensitive state.
- Hydroxyurea can be used in combination with radiotherapy to treat some head and neck cancers.
Sickle cell disease in children
- Initial hydroxyurea dose of 15–20mg/kg body weight per day for six months, then dose increases as tolerated.
- Reduces the incidence of vaso-occlusive events, infections, malaria, transfusions, and death.
- Recommended by the WHO and has regulatory approval in US and Europe for adults and children with sickle cell disease.
- Although hydroxyurea has been suggested to prevent stroke in sickle cell disease patients during the COVID-19 pandemic, there are no clinical trials in progress to confirm this (October 2020).
What are the side effects and risks of hydroxyurea?
Cutaneous side effects of long-term hydroxyurea therapy
Skin side effects are probably very common with hydroxyurea, especially with long-term or high-dose use, with studies reporting skin changes in up to 96% of patients when assessed by a dermatologist.
- Hyperpigmentation
- Skin — diffuse or patchy black-grey; onset 4–12 weeks.
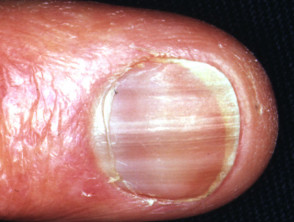
- Nails — generalised/diffuse discolouration, longitudinal melanonychia, transverse pigmented bands, localised discolouration of the lunule, single or multiple nail involvement; onset 6–12 weeks.
- Mucosa — oral and scleral patchy pigmentation.
- Dry skin (xerosis) — up to 100%, can result in acquired ichthyosis.
- Skin atrophy with telangiectases — particularly involving the legs and sun-exposed sites.
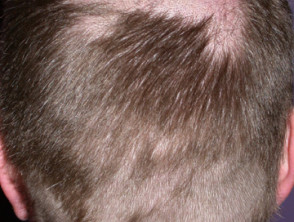
- Alopecia (anagen effluvium) — of the scalp in up to 10% of patients.
- Stomatitis and painful mouth ulcers — can lead to weight loss and tooth decay.
- Hydroxyurea-induced cutaneous ulcer — painful well-defined ulcer(s) on the distal lower leg.
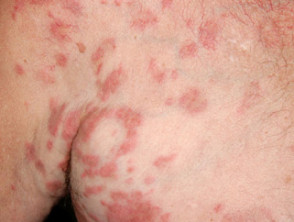
- Drug-induced dermatomyositis — hydroxyurea is the most common cause of this dermatomyositis-like eruption.
- Non-melanoma skin cancers — including actinic keratoses, carcinoma-in-situ, squamous cell carcinoma, and basal cell carcinoma can develop after years of hydroxyurea use.
- Other — many skin and nail effects have been reported in small numbers.
Cutaneous side effects of hydroxyurea
Systemic side effects of hydroxyurea
- Haematological — anaemia, leukopenia, thrombocytopenia
- Gastrointestinal — nausea and vomiting, diarrhoea, transient reversible hepatitis
- Fertility/reproduction — teratogenic, reversible azoospermia
- Drug fever
- Other — uncommon/rare effects involving the lungs, kidneys, and nervous system
Drug interactions
- Live vaccines should be avoided due to the immunosuppressive effects of hydroxyurea [see Immunisation in immunosuppressed dermatology patients].
Monitoring during hydroxyurea treatment
- Full blood count — weekly for the first month then every 2–4 weeks
- Pregnancy test
- Urinalysis
- Liver and renal function tests
- Skin monitoring for ulcers, dermatomyositis-like eruption, squamous dysplasia, and non-melanoma skin cancers.
What are the contraindications to treatment with hydroxyurea?
Absolute contraindications
- Pregnancy
- Lactation
- Drug allergy.
Relative contraindications
- Blood dyscrasias — white cell count <2500 cells/mm3, platelets <100,000/mm3
- Liver, kidney, and cardiopulmonary disease
- Ongoing infection
- Concomitant drugs including cytarabine, methotrexate, and illicit drug use.
Discontinuation of treatment
- Decrease in haemoglobin of 3g/dl or more
- Leukocyte count less than 5000 cells/mm3
- Platelet count less than 150,000 cells/mm3
- Infection.