Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Chin-Yun Lin, Dermatology Registrar, Waikato Hospital, Hamilton, New Zealand, 2012.
Introduction
Uses
Benefits
Other uses
Contraindications
How to use
Side effects at site
Systemic side effects
An intralesional steroid injection involves a corticosteroid such as triamcinolone acetonide injected directly into a lesion on or immediately below the skin.
In New Zealand, triamcinolone injection is marketed as Kenacort-A and is available in 2 strengths: 10 mg per ml (Kenacort-A 10) and 40 mg per ml (Kenacort-A 40). Triamcinolone acetonide is marketed as Kenalog in the USA. Betamethasone injection is marketed as Celestone Chronodose (1 mL) and is not available in New Zealand.
Shorter-acting corticosteroid preparations, such as dexamethasone or betamethasone acetate, are sometimes administered in combination with triamcinolone.
An intralesional steroid injection may be indicated for the following skin conditions:
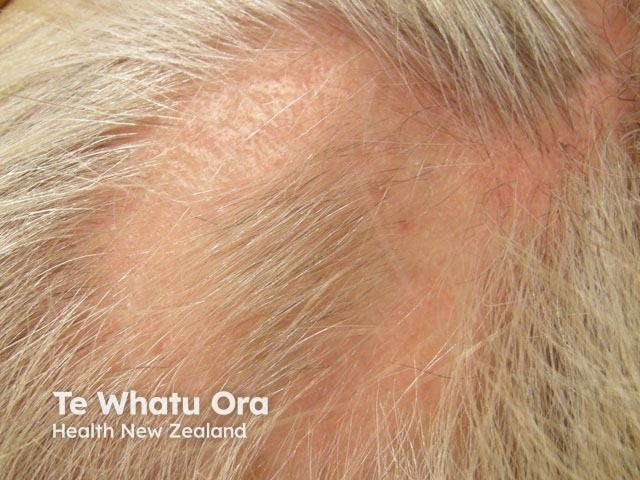
Intralesional steroid injection for alopecia areata
Intralesional administration of corticosteroids treats a dermal inflammatory process directly. In contrast to topical steroids, intralesional steroids:
Triamcinolone injection is also sometimes used intramuscularly (rather than as an intralesional injection) systemically as an alternative to oral corticosteroids, for example for seasonal hay fever, or to treat a chronic skin disorder such as atopic dermatitis or lichen planus.
Typical intramuscular doses are 0.5–1 mg/kg body weight (40–80 mg for a typical adult), which may be repeated every 30 days for 3–6 months.
Triamcinolone injections can also be used in the treatment of tendonitis, arthritis, and synovitis.
Intralesional steroids should not be injected at the site of active skin infection (eg, impetigo or herpes simplex).
They must not be used if there is a known triamcinolone allergy.
When large doses of triamcinolone acetonide are used as an alternative to oral steroids such as prednisone, they are considered to be systemic steroids. These should be avoided in patients with the following disorders:
Intralesional triamcinolone is injected directly into the skin lesion using a fine needle after cleaning the site of injection with alcohol or antiseptic solution. The injection should be intradermal, not subcutaneous, to avoid causing a dent in the skin.
The initial dose per injection site will vary depending on the lesion being treated. Generally, 0.1–0.2 mL is injected per square centimetre of involved skin. The total dose should not normally exceed 1–2 mL per dose. It can be repeated every 4–8 weeks.
The corticosteroid can be full strength (eg, triamcinolone 10 mg/mL or 40 mg/mL) or diluted with normal saline or local anaesthetic. Typical regimes for triamcinolone intralesional injections include:
The injections may be repeated monthly for a few months while the lesions are active.

Intralesional steroid injection
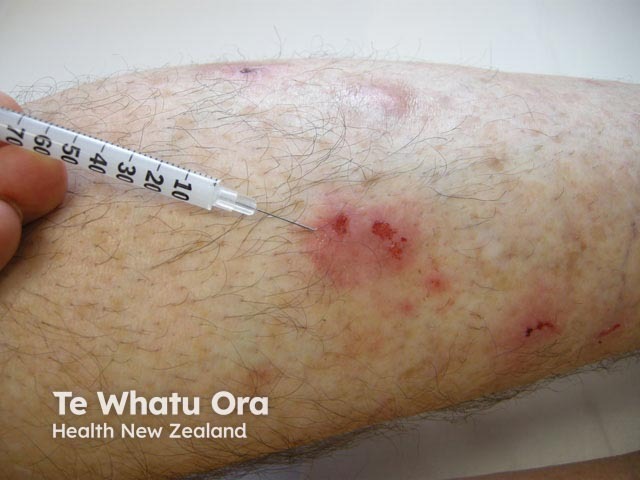
Intralesional steroid injection
Side effects and risks of intralesional triamcinolone may be separated into early and delayed effects.
Early effects tend to be self-limited. They include:
Delayed adverse effects include:
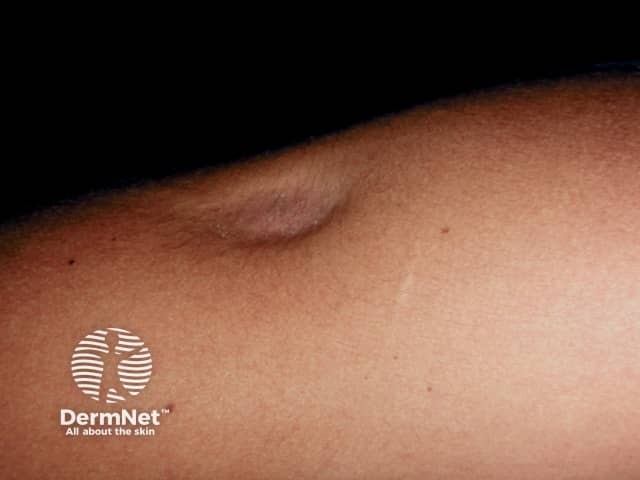
Lipoatrophy
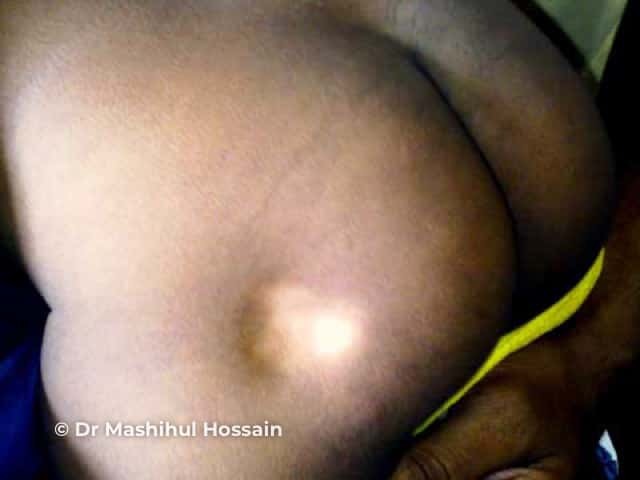
Subcutaneous fat and muscle atrophy with hypopigmentation
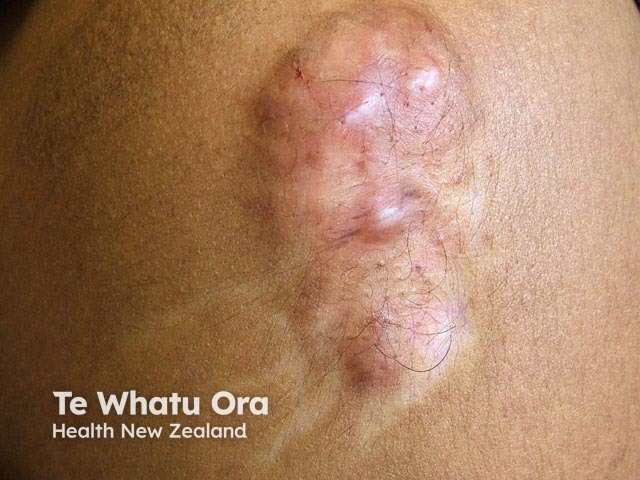
Leukoderma
Allergic reactions are very rare and are dose-independent. They may include local or generalised urticaria (wheal and flare), and in more severe cases, anaphylaxis.
Other systemic side-effects are not likely to follow the intralesional injection of localised skin disease because the dose used is very small.
The following potentially serious conditions have been reported from intramuscular injection of large doses of triamcinolone acetonide.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).