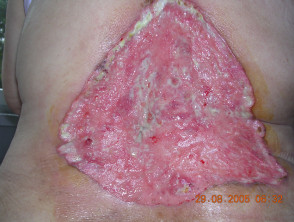
Advanced basal cell carcinoma
Basal cell carcinoma (BCC) is usually curable if the lesions are restricted to a small area of the skin. However, in rare cases, the lesions can become disfiguring, invade surrounding tissue, or metastasise. In these instances of advanced BCC, the disease cannot be effectively treated with the standard treatments of surgery or radiation.
What is vismodegib?
On January 30, 2012, the US Food and Drugs Administration (FDA) approved vismodegib (Erivedge®, made by Genentech, Inc. USA) for the treatment of adults with metastatic basal cell carcinoma. It is not currently funded by PHARMAC in New Zealand (Aug 2016).
Vismodegib is intended for use in adult patients with locally advanced basal cell cancer who are not candidates for surgery or radiation and patients whose cancer has metastasized.
This is the first FDA-approved drug for use in advanced forms of BCC, one of the most common skin cancers.
Advanced basal cell carcinoma
How is vismodegib administered?
- Vismodegib is supplied as a capsule for oral administration.
- The recommended initial dose is 150 mg taken orally once daily until disease progression ceases or until there is an unacceptable amount of toxicity.
- If a scheduled capsule dose is missed, that dose should not be ‘made up for’; resume taking capsules with the next scheduled dose.
- Vismodegib may be taken with or without food.
- Patients are advised to swallow capsules whole and not to crush or open the capsules.
- Capsules should be stored at room temperature between 68°F to 77°F (20°C to 25°C).
How does vismodegib act?
- Vismodegib is an orally administered drug that is designed to selectively inhibit abnormal signalling in the Hedgehog (Hh) pathway, which is an underlying molecular driver of BCC.
- Vismodegib binds to and inhibits Smoothened, a transmembrane protein involved in Hedgehog signal transduction.
- The Hh pathway promotes cellular development and division in several cell types, both by direct cellular activation and by secondary activation of multiple pathways of tissue generation, including angiogenesis (new blood vessel formation) and tissue growth.
- The Hh pathway is typically over-activated in BCC through down-regulation of Hedgehog signal inhibition.
Link to key clinical-trial evidence about vismodegib
Potential drug interactions with vismodegib
- Systemic exposure to vismodegib and incidence of adverse events may be increased when the drug is co-administered with clarithromycin, erythromycin or azithromycin, antibiotics that inhibit the efflux transporter P-glycoprotein.
- Vismodegib does not inhibit or induce CYP450 enzymes.
- Drugs that alter the pH of the upper gastrointestinal tract (e.g. proton pump inhibitors, H2-receptor antagonists, and antacids) may alter the solubility of vismodegib and reduce its bioavailability.
- No formal clinical study has been conducted to evaluate the effect of gastric pH-altering agents on the efficacy of vismodegib.
- Vismodegib does not interact with oral contraceptives.
Adverse events associated with vismodegib
The most common adverse reactions (having an incidence of 10% or more) associated with vismodegib in clinical trials are:
- muscle spasms
- hair loss due to drug-induced alopecia
- altered taste sensation
- weight loss
- fatigue
- nausea
- diarrhoea
- decreased appetite.
The following adverse drug reactions have been identified during post-approval use of vismodegib based on case reports and investigator-initiated studies:
- premature fusion of epiphyses (growth plates)
- precocious puberty.
The use of vismodegib in particular populations
Pregnant women
- Vismodegib has been approved with a boxed warning stating that use of this drug can result in embryo-fetal death or severe birth defects when administered during pregnancy.
- Vismodegib can cause severe birth defects or stillbirth.
- Pregnancy status must be verified before the initiation of treatment with vismodegib.
- A pregnancy test must be done in women of childbearing potential within seven days before starting vismodegib.
- Women of childbearing potential need to be advised of the need for two reliable methods of contraception (including a barrier method) or complete abstinence from sexual intercourse.
- In order to avoid pregnancy, highly effective birth control is advised before starting vismodegib, during treatment, and for 24 months after the last dose of the drug, due to the potential to cause serious developmental defects in breast-fed infants and children (note: this time was extended from an earlier time of 7 months).
- All male patients taking vismodegib, even those with a prior vasectomy, should use condoms with spermicide, during sexual intercourse with female partners while taking vismodegib and for at least two months after the last drug dose.
Nursing mothers
- There is a potential for serious adverse reactions from vismodegib in nursing infants.
- Female patients on vismodegib must not breastfeed.
Blood donors
- Patients must not donate blood or blood products while receiving vismodegib and for at least 24 months after the last drug dose.
Children
- The safety and effectiveness of vismodegib have not been established in children. Vismodegib is not approved for paediatric use.
- Cases of premature fusion of the epiphyses (growth plates) have been reported in children with the use of vismodegib.
Older people
- Clinical studies of vismodegib did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients or to rule out an increased frequency of adverse events.
- No dose adjustment is required in patients ≥ 65 year years of age.
Patients with hepatic impairment
- The pharmacokinetics, safety and tolerability of vismodegib were evaluated in patients with mild, moderate or severe hepatic impairment in a dedicated clinical study, following multiple doses of vismodegib.
- Results demonstrated no impact of hepatic impairment on the pharmacokinetics of vismodegib.
- No dose adjustment is required in patients with mild, moderate or severe hepatic impairment.
Patients with renal impairment
- Results of a population pharmacokinetic analysis demonstrated no impact of renal impairment on the pharmacokinetics of vismodegib.
- No dose adjustment is required in patients with renal impairment.
Effect of cardiac electrophysiology
In a study in 60 healthy subjects, therapeutic doses of vismodegib did not have a significant effect on the QTc interval (a measure of the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle).
Future considerations for vismodegib
Roche and Genentech are currently evaluating vismodegib in a phase 2 trial for patients with operable forms of BCC.
New Zealand approved datasheets are the official source of information for these prescription medicines, including approved uses and risk information. Check the individual New Zealand datasheet on the Medsafe website.