What is adjuvant therapy?
Adjuvant therapy is defined as a treatment given after the initial therapy. In the case of cutaneous melanoma, adjuvant therapy is a form of treatment considered after complete surgical excision of the melanoma (the first-line treatment) [1].
Adjuvant immunotherapy aims to eliminate residual microscopic melanoma cells via the immune system, to reduce the risk of future cancer recurrence, and to improve the overall chance of cure [1].
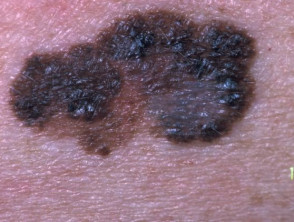
Primary melanoma
Who uses adjuvant therapies?
Indications for consideration of adjuvant therapies for cutaneous melanoma include [1–3]:
- Unfavourable localised disease — intermediate-thickness melanoma (Breslow thickness > 1 mm) with or without additional pathological prognostic factors such as ulceration or increased mitotic rate
- Stage III metastatic melanoma (this is when there is histological evidence of melanoma in regional lymph node/s, in transit, or satellite metastasis)
- Localised melanoma at high risk for local recurrence due to inadequate excision margins or perineural invasion (also termed neurotropism).
What are adjuvant therapies for melanoma?
Immunotherapy and targeted cancer therapy against driver mutations have proven a benefit in stage IV metastatic melanoma (when distant metastases have been detected). Adjuvant therapy is also reported to reduce the relative risk of recurrence by 40% in stage III metastatic melanoma with a 15–25% absolute reduction in local and distant recurrence risk. Overall survival data are not yet reported for immunotherapy, but significant overall survival benefits are seen with adjuvant-combined dabrafenib and trametinib in BRAF–mutant stage III metastatic melanoma.
The results of adjuvant treatment have been published using immunotherapy, targeted therapy, or radiotherapy that has been given for up to a total of 12 months.
Immunotherapy
There are two classes of immune checkpoint inhibitors, as of 2019.
- The cytotoxic T-lymphocyte-associated protein 4 (CTLA4) antibody, ipilimumab (Yervoy®) [4].
- Programmed cell death protein 1 (PD1) antibodies:
- Nivolumab (Opdivo®) [4]
- Pembrolizumab (Keytruda®) [5].
Immunotherapies are given by intravenous infusion every three weeks [4].
Targeted therapies
Targeted therapies block the mitogen-activated protein (MAP) kinase pathway [6].
- BRAF inhibitors include dabrafenib, vemurafenib, and encorafenib.
- MEK inhibitors include trametinib, cobimetinib, and binimetinib.
BRAF inhibitors are administered orally twice daily; a MEK inhibitor is usually taken orally once daily [8]. A BRAF and MEK inhibitor are usually used together in combination therapy to limit early tumour resistance to single-agent therapy [7].
Radiation therapy
Stereotactic external beam radiotherapy can be considered after therapeutic lymphadenectomy to reduce the rate of nodal recurrence, particularly when there is an extranodal extension of metastatic melanoma [8,9].
What are the contraindications with adjuvant therapies?
Typically, patients with lower risk or thin localised melanoma are not currently offered adjuvant therapies (Stage I/IIA disease) [1,2].
The relative contraindications for the use of immunotherapy include:
- Pregnancy — due to concerns about the potential loss of maternal immunological tolerance to a fetus [10]
- A history of poorly controlled or uncontrolled autoimmune disease — activation of the immune system by immunotherapy might lead to a worsening of the autoimmune condition [11]
- Organ transplantation — there have been isolated reports of rapid rejection of the transplanted organ when checkpoint inhibitors have been used. Immunotherapy may still be considered in renal transplant patients if they are also dialysis candidates [12]
- A serious or life-threatening adverse reaction to previous immunotherapy [13].
Targeted therapy using BRAF inhibitors is contraindicated for patients without the BRAF V600 driver mutation [7].
What is neoadjuvant therapy?
Unlike adjuvant therapy, which is given after surgery to remove melanoma, neoadjuvant therapy is an induction treatment commenced before the excision. It is typically used when the primary melanoma is unresectable (ie, it is a large tumour, the excision defect can’t be closed, or the excision would be unacceptable cosmetically) or when any nodal or visceral melanoma metastases are too bulky to safely remove. The intention of neoadjuvant therapy is to reduce the size of the tumour to allow the surgery to take place [14].
What are the benefits of adjuvant therapy for stage III metastatic melanoma?
Early results of clinical research programmes are demonstrating consistent, clinically meaningful benefits from targeted therapies and immunotherapies in stage III metastatic melanoma when the melanoma has been completely resected.
Immunotherapy
Initial research data on immunotherapy using ipilimumab in stage III metastatic melanoma is from two major studies. The chance of overall survival at 5 years was improved by 28%, and the risk of metastatic melanoma recurrence at 5 years was improved by 24% in those randomised to ipilimumab compared to placebo [15].
- In the CheckMate 238 trial, the recurrence-free survival was reduced by 35% in those randomised to receive nivolumab compared to ipilimumab with reduced serious adverse events [4].
- In the KEYNOTE-054 study, patients randomised to pembrolizumab had a 43% reduction in risk of recurrence or death compared to placebo [5].
Targeted therapy
Studies into BRAF inhibitors and BRAF/MEK dual inhibition for patients with stage III metastatic melanoma were conducted prior to the immunotherapy studies. Key results are listed below. A consistent improvement in the overall survival (OS) of patients was demonstrated with the use of single agent MEK/BRAF inhibition compared to placebo. This benefit was increased with dual inhibition [7,16]. Due to the adverse effects of targeted therapy, one of the components may need to be stopped [17].
| Inhibitor | CR + PR% | Median PFS, Median OS |
| Vemurafenib [18] | 53% | 6.9 months, 13.6 months |
| Dabrafenib [19] | 50% | 6.7 months, 18 months |
| Trametinib [20] | 22% | 4.8 months (PFS only) |
| Dabrafenib + trametinib [7] | 67% | 11 months, 25.1 months |
| Dabrafenib + trametinib versus vemurafenib [16] | 64% | 12.6 months, 25.6 months |
CR: complete response; PR: partial response; CR + PR%: percentage of patients with a partial or complete response; PFS: progression-free survival; OS: overall survival
Adjuvant radiotherapy
Local control of melanoma may be enhanced by adjuvant radiotherapy if the risk of local recurrence remains unacceptably high after surgical excision such as with:
- Inadequate wide excision margins, often due to anatomical or cosmetic constraints around the eye/conjunctiva, nose, lips, or ears [21]
- Satellite lesions
- Neurotropism (perineural invasion) on histology [8,9]
- Desmoplastic melanoma, due to its tendency for local recurrence.
Although randomised controlled trials have not evaluated the benefit of adjuvant radiotherapy, several large observational cohorts have reported positive results with 50–85% improvement in risk of local recurrence. Possible evidence was also observed for decreased nodal recurrence [22–24].
The local inflammatory effects resulting from radiation therapy may provide a synergistic boost to the efficacy of immunotherapy [25,26]. Further research is underway to evaluate its benefits [27,28].
What are the disadvantages of adjuvant therapies?
The comparative benefits of immunotherapy to traditional chemotherapy have been game-changing for the treatment of metastatic melanoma in some patients. However, in many patients, melanoma does not respond to immunotherapy or only partially responds. In addition, targeted therapies are only indicated for those whose melanoma carries the BRAF V600 driver mutation (approximately 40–50% of cutaneous melanoma) [29].
There are questions still to be answered about adjuvant therapy for melanoma.
- Does adjuvant immunotherapy improve overall survival for patients with stage III metastatic melanoma?
- Is it better to give adjuvant immunotherapy for stage III metastatic melanoma or wait until stage IV metastatic melanoma disease has developed?
- If a patient has a BRAF V600 mutation, should we use targeted therapy or immunotherapy first?
- Would adjuvant immunotherapy or targeted therapy reduce the risk of the disease if used at an earlier stage of melanoma? A study is currently recruiting participants to answer this question in stage IIB-C melanoma (2019).
What are the side effects and risks of adjuvant therapies?
Adjuvant treatments are generally well tolerated with comparative greater short-term toxicity with BRAF/MEK combined inhibition compared to immunotherapy; however, there is a risk of permanent toxicity with adjuvant immunotherapy.
Side effects of immunotherapy
Immunotherapy can result in side effects due to immune activation [13,30]. The rates described below are for PD1 inhibitors (nivolumab and pembrolizumab); immune-related adverse events are typically more frequent with CTLA-4 inhibition (ipilimumab).
- Fatigue ~25% on a PD1 inhibitor
- Skin: pruritus, dermatitis, or vitiligo in ~34%
- Gastrointestinal tract: colitis causing diarrhoea in 13% (the risk is greater with the combination of ipilimumab and nivolumab)
- Thyroid dysfunction: hyperthyroidism or hypothyroidism in ~10%
- Arthralgia ~7%
- Hepatitis 5–10%
- Pneumonitis 2–4%
- Myositis
- Acute kidney injury
- Rarely, hypophysitis.
See also Cutaneous adverse effects of checkpoint inhibitors.
Side effects of targeted therapies
Combined dabrafenib/trametinib causes the following adverse effects [17].
- Pyrexia
- Fatigue
- Nausea
- Headache
- Diarrhoea
- Vomiting
- Arthralgia
- Dermatitis
- New cutaneous skin cancer, especially squamous cell carcinoma (a BRAF inhibitor effect)
- Cardiac effects (left ventricular dysfunction)
- Ocular effects (blurred vision, chorioretinopathy)
- Pulmonary effects (interstitial lung disease, pneumonitis).
Side effects of radiotherapy
Acute side effects of radiotherapy may include [31,32]:
- Desquamation
- Erythema
- Organ-related inflammation and oedema (eg, oesophagitis, mucositis, pneumonitis)
- Fatigue.
Late side effects of radiotherapy may include:
- Hypopigmentation or hyperpigmentation
- Telangiectasia
- Epidermal atrophy and fragility
- Alopecia and sweat gland atrophy
- Necrosis of soft tissue, cartilage, or bone
- Radiation-induced malignancy — squamous cell carcinoma, basal cell carcinoma.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).