What is dermatopathology?
Dermatopathology is a subspecialty of pathology.
- Pathology is the study of diseases. It includes the study of the causes, course and progression and the complications that arise from the disease.
- Anatomic pathology, or histopathology, refers to the study of the structural and compositional changes that occur in organs and tissues as a result of disease.
- A pathologist is a doctor trained in anatomic pathology that examines, describes and interprets pathological specimens to arrive at a specific finding or diagnosis.
- Dermatopathology is the study and description of structural and compositional changes that occur in skin disease.
From a practical point of view, dermatopathology involves the microscopic examination, description and interpretation of biopsy specimens obtained from the skin. This is usually carried out by a general pathologist (who may or may not have had specific training in dermatopathology) or by a dermatopathologist (a doctor trained specifically in dermatopathology, but who may not have fully trained in anatomic pathology). Dermatopathologists often have training in clinical dermatology.
The interpretation of skin specimens can be complicated and difficult, as many diverse inflammatory skin diseases share the same basic inflammatory process or pattern. The final diagnosis requires clinical input and clinicopathological correlation.
How are skin biopsy specimens examined?
Skin biopsy specimens are processed and then stained with Haematoxylin and Eosin (H&E). Eosin is acidic in nature and stains basic / alkaline / acidophilic structures red/pink. Haematoxylin is alkaline, and stains acidic / basophilic structures (eg, deoxynucleic acid, ribonucleic acid within cell nuclei) blue. Depending on the observed dermatopathological pattern present and/or the clinical features, special stains may be requested to identify agents causing the condition (e.g. bacteria or fungi), specific substances deposited in the skin (e.g. amyloid, iron or melanin) or specific markers to identify the origin, nature and distribution of cells in the specimen being examined.
The specimen is systematically examined by looking at the structure of the epidermis, dermis, subcutis, fascia and underlying structures. Based on the findings, the pathologist may come up with a definitive diagnosis, or list several possible explanations, creating a differential diagnosis. The integration of clinical information in conjunction with the pathological findings generates the final diagnosis, or list several possible explanations, creating a differential diagnosis. The integration of clinical information in conjunction with the pathological findings generates the final diagnosis.
Potential errors in diagnosis
Pathologists depend on the clinician supplying a good history and differential diagnosis, and their job is easier with a large biopsy sample than with a small one. Even then, the sample may not be representative of the disease as a whole.
- The biopsy may have been taken from the wrong lesion.
- The biopsy may not contain diagnostic material.
- The biopsy may be fragmented or crushed.
- There may be processing errors.
- Incorrect diagnosis may arise because of lack information on the request form.
- The microscopy may appear normal despite quite obvious clinical disease.
- Changes may be too subtle to diagnose if the lesion is very early in its development.
- Secondary changes obscure primary pathology. These include excoriation, ulceration, healing, infection, necrosis and fibrosis.
- The thin section examined by the pathologist may not contain any part of the lesion present in another portion of the original specimen. In this case, a smaller biopsy sample might have led to the diagnosis.
- Dense cellular infiltration may obscure the presence of another pathological feature, preventing its identification.
- Two quite different skin diseases might appear similar under the microscope.
Common inflammatory skin diseases
Histological patterns of eczema, psoriasis, lichen planus, bullous pemphigoid, vasculitis and granuloma annulare are described below.
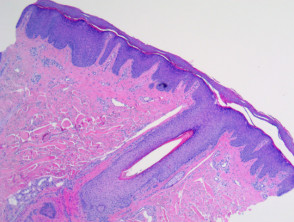
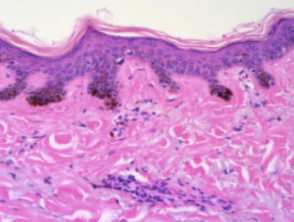
Eczema/dermatitis
The histological features of eczema are:
-
- Spongiosis in acute eczema with associated lymphocyte exocytosis
- Acanthosis in chronic eczema
- Parakeratosis and perivascular lymphohistiocytic infiltrate
- Excoriation and signs of rubbing (irregular acanthosis and perpendicular orientation of collagen in dermal papillae) in chronic cases (lichen simplex).
Pathology of eczema/dermatitis
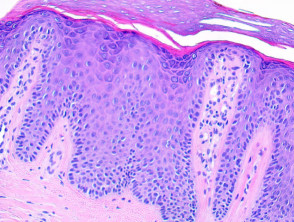
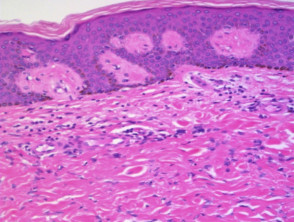
Psoriasis
Typical pathological features of chronic plaque psoriasis are:
-
- Hyperkeratosis – mainly parakeratosis, some orthokeratosis
- Neutrophils in stratum corneum and squamous cell layer
- Hypogranulosis
- Epidermis is thin over dermal papillae
- Regular acanthosis, often with clubbed rete ridges
- Relatively little spongiosis
- Dilated capillaries in dermal papillae
- Perivascular lymphohistiocytic infiltrate.
Pathology of psoriasis
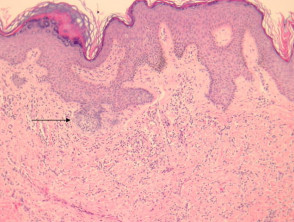
Lichen planus
The histological features of lichen planus are:
-
- Orthokeratosis
- Hypergranulosis
- Irregular acanthosis with saw-toothed rete ridges (older lesions)
- Colloid bodies in lower epidermis and upper dermis
- Liquefaction degeneration of the basal layer
- Lichenoid lymphohistiocytic infiltrate in upper dermis (interface dermatitis) and sometimes within the epidermis
- Melanin incontinence.
Pathology of lichen planus
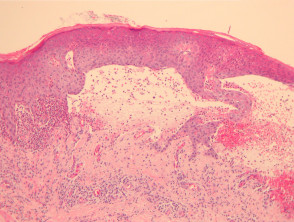
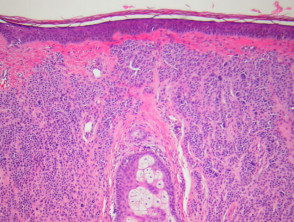
Bullous pemphigoid
The histological features of bullous pemphigoid are:
-
- Subepidermal blister
- Viable roof over new blister, necrotic over an old blister
- Variable perivascular infiltrate (lymphocytes, histiocytes, eosinophils)
- Pre-bullous lesions may show spongiosis with eosinophil exocytosis (eosinophilic spongiosis).
Pathology of bullous pemphigoid
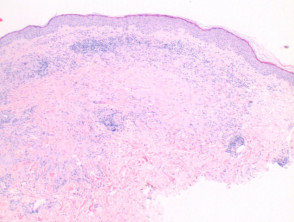
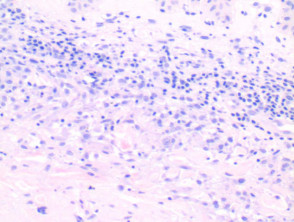
Vasculitis
Note: vascular damage can be a secondary feature of conditions that are not primarily a vasculitis.
The histological features of leucocytoclastic (small vessel) vasculitis are:
-
- Vessel wall damage – necrosis, hyalinisation, fibrin
- Invasion of inflammatory cells into vessel walls
- Red cell extravasation
- Nuclear dust from leucocytoclasia of neutrophils
- Severe cases may show ischaemic necrosis of the epidermis.
Pathology of leucocytoclastic vasculitis
Granuloma annulare
The histological features of granuloma annulare are:
-
- Normal epidermis
- Central foci of dermal collagen degeneration (necrobiosis) and mucin accumulation
- Palisading of histiocytes
- Multinucleate giant cells
- Single-filing of inflammatory cells between collagen bundles (‘busy’ dermis).
Pathology of granuloma annulare
Common cutaneous tumours
- Histology of the common cutaneous tumours seborrhoeic keratosis, basal cell carcinoma, squamous cell carcinoma in situ (intraepidermal carcinoma), squamous cell carcinoma, cysts, lentigo, melanocytic naevus, melanoma and dermatofibroma are described below.
Seborrhoeic keratosis
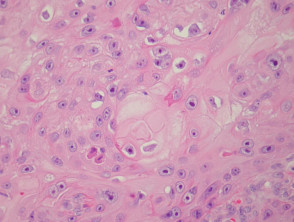
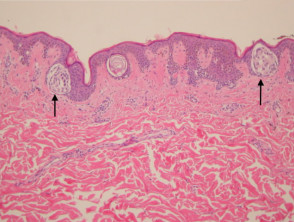
The histological features of seborrhoeic keratosis may be quite varied and can overlap with solar lentigo, but typically show:
- Hyperkeratosis, papillomatosis, acanthosis
- Basaloid keratinocytes
- Horn cysts
- Abundant melanin in basal layer or throughout epidermis
- Sharp demarcation of base of epidermal hyperplasia
- Largely located above the surrounding epidermis
Irritated seborrhoeic keratoses may show many features suggestive of malignancy, and can be difficult at times to differentiate from squamous carcinoma.
Pathology of seborrhoeic keratosis and solar lentigo
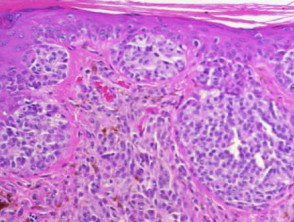
Basal cell carcinoma
The histological features of basal cell carcinoma are typically:
-
- Cohesive nests of basaloid tumour cells (sometimes with a small amount of squamous differentiation)
- Peripheral palisading of nuclei at the margins of cell nests
- Retraction artefact (clefts) around cell nests
- Variable inflammatory infiltrate and ulceration.
Pathology of basal cell carcinoma
Actinic keratosis
The histological features of actinic keratosis are:
-
- Hyperkeratosis and/or ulceration
- Columns of parakeratosis, overlying atypical keratinocytes, separated by areas of orthokeratosis
- Basal atypical keratinocytes with varying degrees of overlying loss of maturation, hyperchromatism, pleomorphism, increased and abnormal mitoses, dyskeratosis – full thickness change may be called ‘Bowenoid actinic keratosis’
- Variable superficial perivascular or lichenoid chronic inflammatory infiltrate
- Solar elastosis.
Pathology of actinic keratosis
In situ squamous cell carcinoma
The histological features of SCC in situ (intraepidermal carcinoma) show extensive overlap with actinic keratosis and are:
-
- Hyperkeratosis, parakeratosis
- Acanthosis
- Full thickness epidermal involvement by atypical keratinocytes, with pale vacuolated or multinucleated cells
- In some lesions, pagetoid spread at the margins.
Pathology of squamous cell carcinoma in situ (intraepidermal carcinoma)
Invasive squamous cell carcinoma
Histological features of invasive squamous cell carcinomas can vary, but in general are:
-
- Proliferation of atypical keratinocytes
- Invasion of dermis
- Variable degrees of keratinisation, sometimes squamous eddies or keratin pearls.
Pathology of squamous cell carcinoma
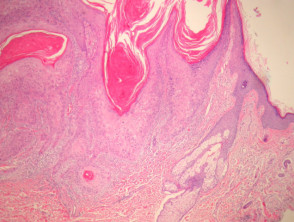
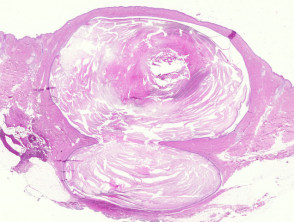
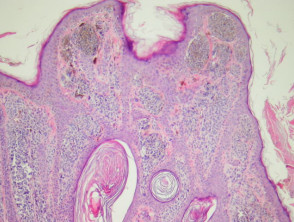
Epidermoid and pilar cysts
The histological features of epidermal inclusion cysts (epidermoid cysts) are:
- Cyst lined by squamous epithelium, sometimes flattened, with a granular layer
- Lamellated keratin within cyst
Dermoid cysts differ by showing hair follicles and sebaceous glands in the wall and hair shafts in the contents
- Milia are very small epidermoid cysts
Trichilemmal (pilar) cysts show:
-
- Squamous lining but no granular layer
- Dense keratin content
- Frequent calcification.
Pathology of cysts
Lentigo
The histological features of lentigines are:
- Hyperpigmented elongated rete ridges
- Increased melanocytes.
Melanocytic naevus
The histological features of melanocytic naevi are:
- Variable epidermal changes – atrophy, hyperplasia, papillomatosis, horn cysts
- Nests of melanocytes/naevus cells at the dermo-epidermal junction (junctional naevus) and/or in the dermis (compound naevus, dermal naevus)
- Naevus cells in the epidermis confined to the basal layer, usually at the tips of the rete ridges
- Generally round naevus cells that show decreasing size of both the cells and the cell nests with increasing depth in the dermis (so-called maturation)
- Little inflammation unless traumatised (except halo and dysplastic naevi).
Pathology of melanocytic naevus
Melanoma
The histological features of melanoma differ, depending on the type of tumour, but in general terms show:
- Asymmetrical proliferation of melanocytes
- Atypical melanocytes invading upwards through epidermis and downwards into dermis
- Variable cytological atypia: loss of maturation, pleomorphism, hyperchromatism, increased mitoses, prominent nucleoli.
Pathology of melanoma
Dermatofibroma
The histological features of dermatofibroma are:
- Epidermal hyperplasia (sometimes mimicking basal cell carcinoma)
- Hyperpigmented basal layer
- Circumscribed but poorly demarcated proliferation of spindled fibroblasts
- Histiocytes and few giant cells
- Variable amounts of collagen.
Pathology of dermatofibroma