Abstract
Introduction
Hidradenitis suppurativa (HS) is a debilitating and distressing chronic inflammatory skin disease affecting about one in every 150 Australians. It is characterised by recurrent, painful, deep-seated, multiple skin nodules, most commonly located in the axillary, inguinal, perineal and perianal regions, the submammary and intermammary folds, and the buttocks. Complications include abscesses, sinus tracks, fistulae, and scarring. Diagnosis is often delayed for many years and misdiagnosis can result in ineffective and potentially harmful treatment.
Many medical disciplines and services, including general practice, emergency medicine, surgery, infectious diseases, gastroenterology, gynaecology, and mammography services, are well placed to recognise HS and to refer patients to dermatologists for early effective treatment.
Main recommendations
Comprehensive care of HS should include encouragement of smoking cessation and weight control, wound care, and management of pain, itch and psychosocial health. Topical therapies alone may be effective in mild HS. Systemic antibiotics are widely used short-term for the control of acute infective episodes and long-term for their presumed immunomodulatory properties. Many non-antibiotic systemic therapies have been used in HS but none is supported by evidence higher than level IV. Adalimumab is the only biologic registered in Australia and New Zealand for the treatment of HS. Surgery is an effective adjunct to medical treatment for HS. Laser therapy, photodynamic therapy and intense pulsed light can also be useful adjuncts to medical therapy.
Changes in management as a result of the guidelines
Early diagnosis and timely referral to expert care are the key to achieving effective multimodal care of HS.
Introduction
Hidradenitis suppurative (HS) is a debilitating and distressing chronic inflammatory skin disease characterised by recurrent, painful, deep-seated, multiple skin nodules. They are most commonly located in areas rich in apocrine glands, namely in the axillary, inguinal, perineal and perianal regions, the submammary and intermammary folds in women, and the buttocks [1]. Subcutaneous inflammation of adjacent nodules can lead to the development of abscesses, sinus tracks, fistulae, and scarring. Complications of severe, chronic HS include soft tissue infection, lymphoedema (especially in the genital area) and, rarely, squamous cell carcinoma (almost exclusively in the anogenital area in men). Scarring and contractures, especially in the axillae, can restrict movement. Anogenital disease can cause strictures of the urethra, anus, and rectum, and sometimes pararectal and paraurethral fistulae. HS can have a profound detrimental effect on quality of life.
Despite the obvious clinical pathology of HS and clear diagnostic criteria, diagnosis is often delayed for many years and misdiagnosis can result in ineffective and potentially harmful treatment [2]. Many medical disciplines and services, including general practice, emergency medicine, surgery, infectious diseases, gastroenterology, gynaecology, and mammography services, are well placed to recognise HS and refer patients to dermatologists for early effective treatment. Once the diagnosis is made, many of these disciplines will continue to care for patients living with the disease.
The evidence base for treatment is sparse. Only 14 randomised controlled trials of HS treatment have been published, covering a wide range of medical and other strategies. Clinical guidelines have been developed by European and Canadian consensus groups [3,4], but they do not necessarily reflect the access to medications and other therapies nor the approach to treatment in Australia and New Zealand. This paper provides an Australasian consensus-based guide to the recognition and treatment of HS.
Methods
The authors, who are dermatologists with a special interest in HS and experience in its treatment, each reviewed and summarised the literature on an allocated topic and, where appropriate, proposed recommendations for treatment. A draft of a consensus statement was discussed by teleconference, subsequently refined, and finalised through a series of reviews. The guidelines consider both the level of evidence [5]; and strength of the recommendation [6].
Background
HS most frequently develops in early adulthood, and onset before puberty or, in women, after menopause, is rare. Females diagnosed with HS outnumber males by a ratio of about 3 to 1. The influence of ethnicity has not been established. Estimates of prevalence range from 0.1% or less to more than 4%, reflecting variation in the settings of studies, methods of ascertainment and diagnostic criteria applied[7]. In face-to-face interviews with 11,433 adult Australian residents using a validated HS screening question, the prevalence of HS was estimated at 0.67% [8].
The inflammatory cascade in HS involves tumour necrosis factor (TNF)-α, interleukin (IL)-17 and interleukin-1β [9]. Recognising HS as an autoinflammatory disease, and not a result of infection, has important clinical implications including the need to address comorbidities such as visceral adiposity, insulin resistance and hormonal factors that can activate or exacerbate the underlying inflammatory mechanisms. About one-third of patients have a family history of HS, and at least 23 potentially pathogenic gene sequence variants have been identified [10]. The microbiome in HS differs significantly from healthy controls in both affected and non-affected skin [11]. Bacterial infection, when it does occur, is likely to be secondary to skin damage and underlying inflammation rather than a primary contributor to pathogenesis.
Behaviours and conditions associated with HS include current or former smoking, elevated body mass index, polycystic ovary syndrome, pyoderma gangrenosum, pilonidal cysts, and other inflammatory conditions including rheumatoid arthritis and inflammatory bowel disease [12].
HS is characterised by the inter-related features of pain, depression, anxiety, disability and impaired quality of life, and comorbid depression is common [13].
Diagnosis and staging
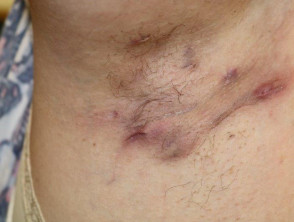
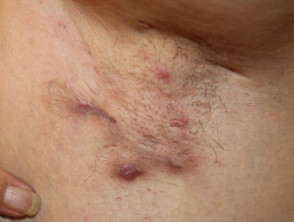
The diagnosis of HS is clinical, and is based on the presence of chronic and recurrent typical lesions, such as nodules, abscesses, bridged scars, draining sinuses and double-ended comedones, in typical locations (Figure) [4]. Symptoms commonly fluctuate over time. Supporting diagnostic criteria, which are not always present, include a positive family history of HS and absence of microbiologically-proven pathogens in the predominant primary lesions. Patients typically have had a poor response to prior treatment, which very often includes multiple short courses of antibiotics and local surgery.
Hidradenitis suppurativa of axilla
Patients with suspected HS should be assessed for predisposing factors such as smoking, being overweight, and having a personal or family history of HS, acne, inflammatory bowel disease, and rare skin disorders such as steatocystoma multiplex. Investigations should be directed by the clinical features of the patient and targeted at excluding other diagnoses, identifying comorbidities, and work-up for current and possible future treatment. Exhaustive investigations are rarely required.
Objective staging and scoring of HS can guide the approach to treatment and assist in monitoring outcomes. The Hurley classification is a static grading system, well established amongst dermatologists. It categorises HS as stage I (single or multiple abscesses, no sinus tracts or scarring), stage II (recurrent single or multiple abscesses with sinus tracts and scarring, and widely separated lesions) or stage III (diffuse or almost diffuse involvement or multiple interconnected tracts and abscesses) [14]. The Hidradenitis Suppurativa Clinical Response (HiSCR) is a dynamic categorical score defined as a ≥ 50% reduction in inflammatory lesion count (sum of abscesses and inflammatory nodules) and no increase in abscesses or draining fistulae when compared with baseline [15]. The routine use of patient-reported outcomes including the Dermatology Life Quality Index (DLQI) and simple visual analogue measures of itch and pain can assist in monitoring the impact of the disease and refining treatment [4].
Treatment
General measures
We recommend that comprehensive care of HS should include encouragement of smoking cessation and weight control, wound care, and management of pain, itch and psychosocial health.
Not smoking is associated with a better response to treatment [16] and a 15% weight reduction in obese patients ameliorates disease severity [17]. Patients report pain to be the most bothersome symptom of HS and a crucial determinant of decreased health-related quality of life [18]. Topical analgesics are sometimes used but are best avoided because of a risk of allergic contact dermatitis. Oral paracetamol and oral nonsteroidal anti-inflammatory drugs are first line agents. Opioid analgesics are second-line therapy but must be used cautiously. Some anticonvulsants and antidepressants relieve neuropathic pain and may be beneficial, especially in patients with comorbid depression [19]. Adalimumab also reduces HS-associated pain [20]. Pruritus is a common and troubling symptom of HS [21]. Topical corticosteroids or oral first-generation antihistamines may sometimes assist.
Wound care in HS aims to manage odour, pain, and exudate (which is sometimes profuse), and minimise bacterial colonisation and secondary infection. Dressing choice is determined by the location of HS lesions, the severity of disease and the morphology of the wound [22]. Patients’ personal experience with wound care frequently provides a guide to continuing care, as they will often have had many years of experience in developing techniques that fulfil their needs. Wound care dressings vary from simple gauze and sanitary napkins to more complex superabsorbent polymer dressings, silver impregnated foams, hydrofibres and calcium alginates with silver. Atraumatic adhesives are required to avoid friction, and should be appropriately shaped for curved anatomical locations.
HS can have a profound impact on quality of life and psychological wellbeing. It is essential to recognise the psychosocial costs of the disease and either provide, or refer for, appropriate support and treatment for consequences such as depression and anxiety.
Topical and intralesional therapy
Topical therapies alone may be effective in mild HS where there are no deep inflammatory lesions, and they are commonly used as adjunctive therapy in more severe disease. Bleach baths and topical disinfectants such as chlorhexidine aim to maintain skin hygiene, reduce bacterial colonisation and potentially suppress the triggers of an aberrant immune response (level of evidence IV) [23].
Resorcinol 15% in aqueous cream can be effective in resolving follicular blockage (level of evidence III) [24]. It should be applied only to the lesions themselves and not more widely, as it can cause a severe peel.
Intralesional corticosteroids, for example triamcinolone acetonide 10 mg/mL, are often used to rapidly reduce acute flares, especially to reduce pain, and to manage non-responsive nodules and sinus tracts, both as monotherapy and as an adjunct to systemic therapies (level of evidence III) [25].
Small studies have demonstrated the efficacy of clindamycin lotion 1% in aqueous cream applied daily (level of evidence IIb) [26,27]. However, use of clindamycin lotion is limited by concerns about the risks of antibiotic resistance.
Systemic antibiotics
Routine culture of HS lesions regularly fails to demonstrate infection, and if bacteria are identified they often comprise normal flora. However, systemic antibiotics are widely used in treatment, both short-term for the control of acute infective episodes and long-term for their presumed immunomodulatory properties.
In the only randomised controlled trial of systemic antibiotics, 16 weeks’ treatment with oral tetracycline 500 mg bd and topical clindamycin 1% bd were equally effective in a range of outcomes including patient global evaluation, soreness, physician global evaluation and counts of abscesses and nodules (level of evidence IIb) [27]. Tetracycline 500 mg bd has been recommended as a first line treatment option in patients with widespread Hurley I or mild Hurley II stage disease, especially when there are no deep inflammatory lesions, for up to 4 months [7]. In practice, tetracycline derivatives including doxycycline and minocycline are preferred as they have more favourable side effect profiles, and tetracycline is unavailable in Australia and New Zealand. Level III evidence supports the efficacy of a combination of clindamycin 300 mg bd and rifampicin 600 mg daily [28]. Clinical experience has indicated that penicillin-based antibiotics may be effective in flares of HS associated with superinfections but have little benefit in managing the primary disease [29].
Dapsone has been used in HS, based on efficacy in patients with mild to moderate disease [30]. Rapid recurrence after stopping treatment suggests that the effect is predominantly anti-inflammatory.
Many dermatologists commence treatment with doxycycline or minocyline 100 mg daily to determine efficacy, increasing to 100 mg twice daily if necessary. If effective, the dose can be reduced to 50 mg daily or less for maintenance. For Hurley Stage II/III disease, a 10- to 12-week course of rifampicin 300 mg bd and clindamycin 300 mg bd (or similar) is used for severe inflammatory flares. It is essential to monitor the effect of treatment, and be mindful of the risks of bacterial resistance and the potential adverse effects of antibiotics.
Non-antibiotic systemic treatments
A wide range of non-antibiotic systemic therapies have been used in HS but none is supported by evidence higher than level IV.
Systemic corticosteroids are widely prescribed for their anti-inflammatory effect, and clinical experience suggests that they are beneficial as rescue therapy in managing flares [31].
The efficacy of acitretin has been demonstrated in small case series, identifying benefit in severe disease as monotherapy [32] and as an adjuvant to standard systemic medications [33].
Isotretinoin has been used to treat HS on the incorrect assumption that the pathogenesis is similar to that of acne vulgaris but is effective in a minority of patients [34].
Clinical experience suggests that antiandrogens and oestrogens may be effective in mild to moderate HS, but the optimal regimens have not been defined [4]. Regimens that have been described include spironolactone 50–200 mg daily, ethinyloestradiol 50 μg and norgestrel 500 μg daily on days 5–25 of each menstrual cycle, and ethinyloestradiol 50 μg and cyproterone acetate 50 mg on days 5–14 of each cycle. Some benefit has also been demonstrated with the 5-alpha-reductase inhibitor finasteride 5 mg/day. Combined oral contraceptives, especially those containing less androgenic third-generation progestins, may be beneficial for women of childbearing age who also seek fertility control [35].
Obesity, insulin resistance and the metabolic syndrome have been implicated in the pathogenesis of HS. In one small case series, metformin titrated to doses up to 1.5 g/day was associated with clinical improvement at 12 weeks [36].
Many other treatments have demonstrated some clinical benefit in small studies, including ciclosporin 3–6 mg/kg/day [37] zinc gluconate 90 mg/day [38], colchicine 0.5 mg bd [39], intramuscular gamma-globulin [40], fumarates [41], oral tacrolimus [42], and botulinum toxin [43].
We recommend that it is reasonable to consider the use of acitretin (unless contraindicated by pregnancy or potential pregnancy) and avoid isotretinoin (unless the patient also has acne). In females, oral antiandrogens with oral contraceptives are options. Metformin, colchicine or zinc gluconate may be helpful as adjuvant therapies. Oral prednisone is a useful short-term rescue medication, and systemic ciclosporin can be used in the medium term. Comorbidities should be considered before initiating any of these treatments, none of which are formally approved for the treatment of HS.
Biologics
Over the last 10 years a number of biological medications have been trialled in HS, including TNF inhibitors (adalimumab, infliximab, etanercept), IL-1 antagonists (anakinra, canakinumab), the IL-17 inhibitor secukinumab, the IL-12/23 inhibitor ustekinumab, and the PDE4 inhibitor apremilast.
There are very few randomised clinical trials (RCTs) using biologicals in HS [44]. Comparison between the reports is challenging because of varied outcome measures, and there are few head-to head-studies. Limited randomised, blinded trials have been conducted measuring the efficacy of specific biologic treatments for HS with validated scoring systems.
The TNF inhibitors infliximab and adalimumab have been assessed in RCTs (level of evidence II). To date, adalimumab is the only biologic registered in Australia and New Zealand for the treatment of HS. Adalimumab had a positive influence on HS scores (HiSCR, DLQI, and pain visual analogue score) and was well tolerated with no new signals of adverse side effects [45,46]. Benefit has also been demonstrated with infliximab [47].
Surgery
Surgery is an effective adjunct to medical treatment for HS but no randomised controlled trials have assessed this approach. The literature consists primarily of retrospective case series describing a variety of strategies in heterogeneous groups of patients, so systematic comparison of surgical techniques is not possible. No formal practical guidelines on the choice of surgical technique in HS have been published.
The largest published case series included 590 consecutive surgically-treated patients in whom excision was most common (68.6%), followed unroofing (deroofing), exteriorisation or curettage (28.5%) and incision and drainage (2.9%) [48]. Resultant wounds were managed by healing by secondary intention (42.4%), primary closure (41.7%), marsupialisation (8.0%), skin grafting (4.9%) or flap (2.2%). Postoperative recurrence occurred in 24.4% of patients, requiring reoperation in 11.7% of all patients. Recurrence risk was increased by younger age, multiple surgical sites and drainage-type procedures. The analysis concluded that well-planned surgical treatment aiming to remove or unroof the area of intractable disease was highly effective in managing this challenging condition.
Patient preference and the size and location of lesions are factors to consider. Our recommendations in the table are based on the existing literature, experience in multidisciplinary teams, conference presentations and communication with colleagues.
| Site/lesion type | Technique | Comments |
|---|---|---|
| Small nodules | Punch excision or deroofing, healing by secondary intention | Aim for early intervention to prevent rupture of blocked, inflamed follicles and development of chronic draining and interconnecting sinus tracts |
| Chronic sinus tracts in flexures (axillae and groin) <200 cm2 area, or extensor surface <100 cm2 | Deroofing surgery, with healing by secondary intention. Remove the roof of the sinus and any fibrotic tissue at base, often to deep dermis or, in chronic lesions, to the fat layer. High volumes of local anaesthetic may be required. Lignocaine 1% and adrenaline can be diluted with plain lignocaine 50:50 if in minor theatre with no anaesthetist present. Consider general anaesthesia for large lesions. | Postoperatively, wash daily with gauze soaked with saline or tap water, apply petroleum jelly to the wound and then an absorbent padded dressing, minimising adhesive sticking plaster to reduce irritation. |
| Chronic sinus tracts on extensor surfaces (for example buttock) or in flexural sites where large size, location or patient preference requires a flap or graft | Tend not to heal well by secondary intention, consider split thickness skin graft or flap | Usually requires general anaesthesia and consultation with a plastic surgeon, general surgeon, or colorectal surgeon if lesions within 1 cm of anus. |
Other physical therapies
Laser therapy, photodynamic therapy, intense pulsed light and hyperbaric oxygen have been investigated in the treatment of HS.
Laser and light-based therapies have the potential to reduce the frequency of painful flares by decreasing the number of hair follicles, sebaceous glands and bacteria in affected areas, and to ablate chronic problematic lesions. Ablative CO2 laser therapy has been used to deroof sinus tracts, vaporise them until no affected tissue is visible, and completely excise them along with perilesional skin [49].
Several small studies have described the efficacy of photodynamic therapy (PDT) in HS [50,51], for example using 5-aminolevulinic acid and a 635-nm light source. PDT is currently used by dermatologists in Australia and New Zealand to manage cutaneous actinic malignancies and its use in HS could be explored further in controlled trials. Two small studies showed that intense pulsed light at 420 nm or 630 nm were effective in reducing the severity of HS lesions and was well tolerated [52,53]. Adjunctive hyperbaric oxygen therapy was associated with benefit in a small pilot study [54].
Clinical experience indicates that physical therapies can potentially improve outcomes but there is a lack of robust evidence and experience with combination therapies. Cost, access and experience with these modalities are barriers to their wider adoption, but encouraging results in case series warrant further investigation.
Future directions
Further efforts are required to educate healthcare professionals and the general public about HS, and to encourage research on its epidemiology and pathophysiology. Many disciplines have an opportunity to identify patients with HS who have not yet been diagnosed accurately, and facilitate their early referral for effective treatment. Despite the scarcity of high-quality evidence to guide treatment, positive outcomes can be achieved with existing therapies, although better information is needed to guide their initial selection, combination and sequencing. More research is needed to develop new treatment strategies.