Introduction
In July 2017, the US Food and Drug Administration (FDA) approved guselkumab (Tremfya™; Janssen Biotech, PA, USA) for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Guselkumab is the first and only approved biological therapy that selectively blocks only interleukin (IL)-23, a cytokine that plays a key role in plaque psoriasis.
Guselkumab received FDA approval based on results from a clinical development programme that included more than 2,000 patients in the Phase III VOYAGE 1, VOYAGE 2, and NAVIGATE studies.
In September 2017, the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending marketing authorisation in the European Union for the use of guselkumab in the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy.
Guselkumab is currently unavailable in New Zealand (as of January 2018).
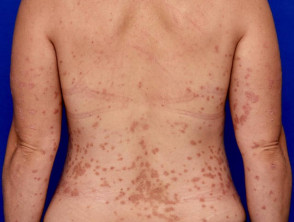
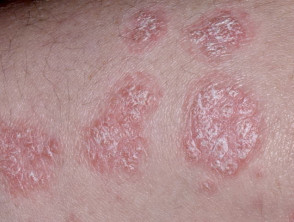
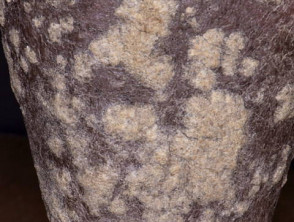
Moderate-to-severe psoriasis suitable for guselkumab
VOYAGE 1 and 2 (guselkumab compared with placebo or adalimumab)
- A total of 1443 patients were randomised to either guselkumab (100 mg at Weeks 0 and 4 and every 8 weeks thereafter), placebo, or adalimumab (80 mg at Week 0 and 40 mg at Week 1, followed by 40 mg every other week thereafter).
- Patients had an Investigator’s Global Assessment (IGA) score of ≥ 3 (moderate) on a 5-point scale of overall disease severity, a Psoriasis Area and Severity Index (PASI) score ≥ 12, and a minimum affected body surface area of 10%.
- Patients with guttate, erythrodermic, or pustular psoriasis were excluded.
- The two co-primary endpoints were (1) the proportion of individuals who achieved an IGA score of 0 (cleared) or 1 (minimal); and (2) the proportion of subjects who achieved at least a 90% reduction from baseline in PASI composite score (PASI 90).
- Both the VOYAGE 1 and 2 trials assessed the responses at Week 16 compared to placebo for the two co-primary endpoints.
- Efficacy results are summarised in Table 1.
Table 1. Efficacy results at Week 16 in adults with plaque psoriasis compared with placebo in VOYAGE 1 and 2.
|
VOYAGE 1 |
VOYAGE 2 |
|||
|
Primary endpoint No. of patients (%) |
Guselkumab (n = 329) |
Placebo (n = 174) |
Guselkumab (n = 496) |
Placebo (n = 248) |
|
IGA response of 0/1 |
280 (85) |
12 (7) |
417 (84) |
21 (8) |
|
PASI 90 response |
241 (73) |
5 (3) |
347 (70) |
6 (2) |
IGA, Investigator's Global Assessment score; PASI 90, 90% reduction from baseline in Psoriasis Area and Severity Index composite score.
Comparison of guselkumab with adalimumab
Table 2 presents the results of an analysis of all the North American sites (US and Canada) in the VOYAGE 1 and 2 trials, demonstrating superiority of guselkumab to adalimumab.
Table 2. Efficacy results for guselkumab versus adalimumab in plaque psoriasis at the North American sites of VOYAGE 1 and 2
|
VOYAGE 1 |
VOYAGE 2 |
|||
|
Endpoint No. of patients (%) |
Guselkumab (n = 115) |
Adalimumab (n =115) |
Guselkumab (n = 160) |
Adalimumab (n= 81) |
|
IGA response of 0 (cleared) or 1 (minimal) |
||||
|
Week 16 |
97 (84) |
70 (61) |
119 (74) |
50 (62) |
|
Week 24 |
97 (84) |
62 (54) |
119 (74) |
46 (57) |
|
Week 48 |
91 (79) |
62 (54) |
NA |
NA |
|
IGA response of 0 (cleared) |
||||
|
Week 24 |
61 (53) |
27 (23) |
76 (48) |
23 (28) |
|
Week 48 |
54 (47) |
28 (24) |
NA |
NA |
|
PASI 75 response |
||||
|
Week 16 |
105 (91) |
80 (70) |
132 (83) |
51 (63) |
|
PASI 90 response |
||||
|
Week 16 |
84 (73) |
47 (44) |
102 (64) |
34 (42) |
|
Week 24 |
92 (80) |
51 (41) |
113 (71) |
41 (51) |
|
Week 48 |
84 (73) |
53 (46) |
NA |
NA |
Maintenance of response
To evaluate maintenance and durability of response in VOYAGE 2, patients randomised to guselkumab at Week 0 who were PASI 90 responders at Week 28 were re-randomised to guselkumab every 8 weeks or placebo. At Week 48, 89% of patients who continued treatment with guselkumab maintained PASI 90 compared to 37% who were re-randomised to placebo.
Patient-reported outcomes
Based on the Psoriasis Symptoms and Signs Diary (PSSD) at Week 16, patients treated with guselkumab showed greater improvements in itch, pain, stinging, burning, and skin tightness compared to placebo, in both VOYAGE 1 and 2 trials. A greater proportion of patients treated with guselkumab compared with adalimumab achieved a PSSD symptom score of 0 (symptom-free) at week 24, in both trials.
Adverse events with guselkumab (VOYAGE 1 and 2)
Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trials may not reflect the rates observed in practice. Table 3 summarises commonly observed adverse events in the VOYAGE 1 and 2 trials.
Table 3. Adverse events occurring in ≥ 1% of patients through to Week 16 in Voyage 1 and Voyage 2 trials
|
Adverse events No. of events (% of patients) |
Guselkumab (n = 283)
|
Adalimumab (n = 196)
|
Placebo (n = 422) No. (%) |
|
Upper respiratory infections |
118 (14.3) |
21 (10.7) |
54 (12.8) |
|
Headache |
38 (4.6) |
2 (1) |
14 (3.3) |
|
Injection site reactions |
37 (4.5) |
15 (7.7) |
12 (2.8) |
|
Arthralgia |
22 (2.7) |
4 (2) |
9 (2.1) |
|
Diarrhoea |
13 (1.6) |
3 (1.5) |
4 (0.9) |
|
Gastroenteritis |
11 (1.3) |
4 (2) |
4 (0.9) |
|
Tinea infection |
9 (1.1) |
0 |
0 |
|
Herpes simplex infection |
9 (1.1) |
0 |
2 (0.5) |
Elevated liver enzymes
Elevated liver enzymes were reported more frequently in the guselkumab group (2.6%) than in the placebo group (1.9%). Of the 21 patients who reported elevated liver enzymes in the guselkumab group, all events except one were mild-to-moderate in severity and none of the events led to discontinuation of the drug.
Safety through Week 48
The frequency of AEs was similar to the safety profile observed during the first 16 weeks of treatment.
NAVIGATE trial (guselkumab compared with ustekinumab)
- In this Phase III, randomised, double-blind study, 871 patients received open-label ustekinumab (45 mg or 90 mg) at Weeks 0 and 4.
- At Week 16, 268 patients with an inadequate response to ustekinumab IGA ≥ 2) were randomised (double-blind) to guselkumab 100 mg or to continue ustekinumab. 585 out of 871 patients (67%) with IGA response of 0 (cleared) or 1 (minimal) at week 16 continued open-label ustekinumab.
- The primary end point was the number of visits at which randomised patients achieved IGA 0/1 and at least a two-grade improvement from Week 28 to Week 40.
- Improvement ≥ 90% or 100% in PASI (PASI 90/100) and Dermatology Life Quality Index (DLQI) of score of O or 1 were also assessed.
- The mean number of visits at which patients achieved an IGA response of 0 or 1 and at least a two-grade improvement during Weeks 28–40 was significantly greater in the guselkumab group versus the randomised ustekinumab group (1.5 vs. 0.7; p < 0.001).
- A greater proportion of patients in the guselkumab group compared with the ustekinumab group achieved an IGA response of 0 or 1 and at least a two-grade improvement at week 28 and week 52.
- A greater proportion of patients treated with guselkumab compared with ustekinumab achieved PASI 90, PASI 100, and DLQI score of 0 or 1 at Week 52.
- After Week 16, 64.4% of patients in the guselkumab group and 55.6% in the ustekinumab group had at least one AE; infections were the most frequent AE type.
- Overall, 6.7% (n = 9) of patients in the guselkumab group had at least one serious AE compared with 4.5% (n = 6) for the ustekinumab group.
- Tables 4 and 5 are a summary of the key results and AEs from the NAVIGATE trial.
Table 4. Efficacy results of guselkumab versus ustekinumab in NAVIGATE
|
Efficacy parameter |
Guselkumab (n = 135) |
Ustekinumab (n = 133) |
P value |
|
Mean number of visits at which patients achieved an IGA response of 0 or 1 and at least a two-grade improvement (relative to Week 16) from Week 28 through to Week 40 |
1.5 |
0.7 |
p≤ 0.001 |
|
Mean number of visits at which patients achieved an IGA response of 0 from Week 28 through to Week 40 |
0.9 |
0.4 |
p≤ 0.001 |
|
Mean number of visits at which patients achieved a PASI 90 from Week 28 through to Week 40 |
2.2 |
1.1 |
p ≤ 0.001 |
|
Number of patients with an IGA response of 0 or 1 and ≥ 2-grade improvement (relative to Week 16) at Week 28 |
42 |
19 |
p ≤ 0.001 |
|
Number of patients with a PASI 90 response at Week 28 |
65 |
30 |
p ≤ 0.001 |
|
Number of patients with DLQI score > 1 at Week 16, |
103 |
105 |
p = 0.002 |
|
Number of patients with DLQI score of 0 or 1 at Week 52 |
40 |
20 |
|
|
Number of patients with a PSSD score > 0 at Week 16 |
123 |
126 |
|
|
Number of patients with a PSSD score of 0 at Week 52 |
25 |
12 |
p <0.05 |
DLQI, Dermatology Life Quality Index; Investigator's Global Assessment score; PASI 90, 90% reduction from baseline in Psoriasis Area and Severity Index composite score; PSSD, Psoriasis Symptoms and Signs Diary.
Table 5. Adverse events during Weeks 1–60 in patients randomised to guselkumab or ustekinumab in NAVIGATE
|
Adverse event No. of events (% of patients) |
Guselkumab (n = 135) |
Ustekinumab (n = 133) |
|
Infections |
57 (41.5%) |
47 (35.3%) |
|
Nasopharyngitis |
23 (17%) |
23 (17.3%) |
|
Respiratory tract infections |
15 (11.1%) |
11 (8.3%) |
|
Injection site reaction |
7 (1.1 %) |
0 |
|
Cardiovascular event |
2 (1.5 %) |
1 (0.8%) |
|
Malignancy |
2 (1.5%) |
0 |
Guselkumab — future potential
- Guselkumab has generated encouraging data for efficacy and safety in the treatment of moderate-to-severe chronic plaque psoriasis.
- Results from Phase III clinical trials suggest that guselkumab is superior to placebo at Week 16 and is better able to clear or almost clear psoriasis plaques compared with adalimumab. The effects were maintained through Week 48.
- Phase III studies of guselkumab report some of the highest PASI 90 rates (at Week 16, 73.3% in VOYAGE 1 and 70.0% in VOYAGE 2) to date. The potential of guselkumab to achieve a PASI 90 response in ≥ 70% of patients suggests that guselkumab may have a significant positive impact on patient's quality of life.
- Results from the NAVIGATE trial established that guselkumab, had substantial efficacy in adults with moderate-to-severe psoriasis and was more effective than ustekinumab in patients with an inadequate response to ustekinumab.
- Potential reasons for the superior efficacy of guselkumab compared with ustekinumab include the central role of IL-23 in the pathogenesis of psoriasis, a more potent blockade of IL-23 with guselkumab, more frequent and higher dosing with guselkumab, and/or enhanced Th17 responses in the setting of the inhibition of IL-12.
- The favorable AE profile of guselkumab observed in Phase III studies suggests that IL-23 may be a more psoriasis-specific cytokine compared to other cytokines such as tumour necrosis factor-alpha and IL-17.
- Overall, targeting IL-23, a key cytokine driving various other effector cytokines, may be responsible for the high efficacy and durable responses seen with guselkumab up to Week 48.
- Further long-term studies are needed to understand the long-term efficacy and safety of guselkumab.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).