Introduction
In July 2015 the US FDA (Food and Drug Administration) approved sonidegib (Odomzo®; Novartis, New Jersey, USA) for the treatment of adult patients with locally advanced basal cell carcinoma (BCC).
The European Commission approved the use of sonidegib for basal cell carcinoma in August 2015. In June 2016, sonidegib was granted marketing authorisation in Switzerland for treatment of adults with advanced basal cell carcinoma. The drug is also approved in Australia. Additional regulatory submissions are being reviewed by health authorities worldwide.
What is sonidegib used for?
Sonidegib is indicated for the treatment of adult patients with locally advanced basal cell carcinoma that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy.
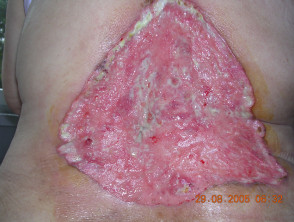
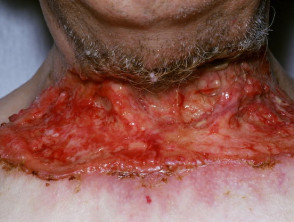
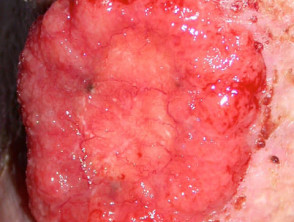
Advanced basal cell carcinoma
How does sonidegib work?
- Sonidegib is an inhibitor of the Hedgehog pathway.
- It binds to and inhibits Smoothened, a transmembrane protein involved in Hedgehog signal transduction, which plays a critical role in stem cell maintenance and tissue repair.
Dosage and administration of sonidegib
- Sonidegib is administered orally.
- It is available as an opaque pink-coloured capsule.
- The recommended dose is 200 mg once daily on an empty stomach, at least 1 hour before or 2 hours after a meal, administered until disease progression or unacceptable toxicity.
- Prior to initiating sonidegib, ensure the patient is not pregnant.
- Sonidegib can cause embryo-fetal death or severe birth defects when administered to a pregnant woman.
- Females of reproductive potential should be advised to use effective contraception during treatment with sonidegib and for at least 20 months after the last dose.
- In all patients, serum creatine kinase (CK) levels must be carried out prior to initiating sonidegib because of potential musculoskeletal adverse effects.
- The drug should be permanently discontinued if serum CK elevation is greater than 2.5 times upper normal limit with worsening renal function.
- The dose of sonidegib should be modified in the case of severe musculoskeletal adverse reactions.
- If a dose of sonidegib is missed, dosing should be resumed with the next scheduled dose.
Link to key clinical-trial evidence about sonidegib
Potential drug interactions with sonidegib
- Sonidegib is primarily metabolised by the hepatic enzymes CYP3A (cytochrome P450 family of oxidising enzymes).
- Concomitant administration of sonidegib with strong and moderate CYP3A inhibitors, including saquinavir, telithromycin, ketoconazole, itraconazole, voriconazole, posaconazole nefazodone, diltiazem, atazanavir and fluconazole should, therefore, be avoided.
- Concomitant administration of sonidegib with strong and moderate CYP3A inducers, including carbamazepine, efavirenz, modafinil, phenobarbital, phenytoin, rifabutin, rifampin and St. John’s Wort should also be avoided.
What are the adverse effects of sonidegib?
The most common (≥ 10%) adverse reactions associated with sonidegib treatment in clinical trials were:
- muscle spasms
- alopecia
- dysgeusia (abnormal taste)
- fatigue
- nausea
- musculoskeletal pain
- diarrhoea
- decreased weight
- decreased appetite
- myalgia (muscle aches)
- abdominal pain
- headache
- vomiting
- pruritus
Warnings and precautions
- In animal reproduction studies, sonidegib was embryotoxic, fetotoxic, and teratogenic at maternal exposures below the recommended human dose of 200 mg.
- Sonidegib can cause embryo-fetal death or severe birth defects when administered to a pregnant woman.
- Female patients should use effective contraception during treatment with sonidegib and for at least 20 months after the last dose.
- Male patients with female partners should be advised to use condoms, even after a vasectomy, during treatment with sonidegib and for at least 8 months after the last dose to avoid potential drug exposure in pregnant females or females of reproductive potential.
- Patients should be advised not to donate blood or blood products while taking sonidegib and for at least 20 months after the last dose of sonidegib because their blood or blood products might be given to a female of reproductive potential.
- Musculoskeletal adverse reactions, accompanied by serum creatine kinase (CK) elevations, may occur with sonidegib and other drugs which inhibit the hedgehog pathway.
- Baseline serum CK and creatinine levels should be measured prior to initiating sonidegib.
- Serum creatinine and CK levels should be measured at least weekly in patients with musculoskeletal adverse reactions with concurrent serum CK elevation greater than 2.5 times upper limit of normal, until resolution of clinical signs and symptoms.
- Temporary dose interruption or discontinuation may be required in the face of musculoskeletal adverse reactions or serum CK elevation.
Use of sonidegib in pregnancy
- There are no available data on the use of sonidegib in pregnant women.
- Data from animal reproduction studies indicate that sonidegib can cause fetal harm when administered to a pregnant woman.
Use of sonidegib in nursing mothers
- No data are available regarding the presence of sonidegib in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production.
- Women should be advised to discontinue breastfeeding during treatment and for 20 months after the last dose.
Paediatric use of sonidegib
The safety and effectiveness of sonidegib have not been established in children (< 18 years of age).
Geriatric use of sonidegib
- In clinical trials with sonidegib, 54% of patients were 65 years and older, and 28% were 75 years and older.
- No overall differences in effectiveness were observed between these patients and younger patients.
- However, there was a higher incidence of serious adverse events requiring dose interruption or discontinuation in patients ≥65 years, compared with younger patients.
Hepatic impairment and sonidegib
- Mild (Child-Pugh class A; the Child-Pugh classification is used to assess the prognosis of chronic liver disease), moderate (Child-Pugh class B), or severe (Child-Pugh class C) hepatic impairment had no clinically meaningful effect on sonidegib exposure as compared to subjects with normal hepatic function.
Renal impairment and sonidegib
- In clinical trials mild renal impairment (creatinine clearance 60 to 89 mL/min) and moderate renal impairment (creatinine clearance 30 to 59 mL/min) had no effect on sonidegib steady-state exposure as compared to patients with normal renal function (Ccr ≥ 90 mL/min).
Effect of sonidegib on age, sex, weight and race
- Age, body weight, or sex had no clinically meaningful effect on sonidegib steady-state exposure.
- A cross-study comparison has suggested that the geometric mean AUCinf (area under the plasma concentration versus time curve from zero to infinity) of sonidegib is 1.7-fold higher in Japanese healthy subjects compared to Western healthy subjects (Whites and Blacks) following a single 200 mg dose.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).