DermNet provides Google Translate, a free machine translation service. Note that this may not provide an exact translation in all languages
Home Common skin lesions Surgical procedures CME
Common skin lesions
Surgical procedures
Created 2008.
Learning objectives
Describe:
- Shave/curettage of superficial skin lesions
- Diagnostic punch biopsy
- Excision and closure of a small skin lesion
- The indications for electrosurgery
Introduction
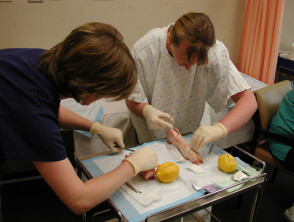
Minor surgical procedures to remove skin lesions can be learned quite easily. Do not rely on this course, which simply describes some procedures in outline. It's a good idea to practice your technique using firm fruit and meat; pigs' trotters or pig's flank may be used.
Practising excisions on pigs' trotters
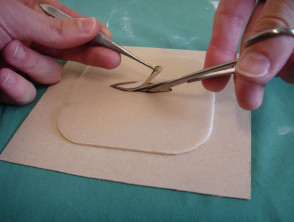
Alternatively, the self-adhesive synthetic dressing Duoderm CGF® might prove adequate as a substitute to practice suturing.*
- Remove the backing paper, except for a one-centimetre rim
- Sprinkle talcum powder on the sticky part to make it unsticky
- Remove the rim of backing paper, and stick the Duoderm to a piece of card
- Draw a fusiform shape, and cut this out
- Suture the artificial wound
Practising suturing on Duoderm CGF®
If you don't have QuickTime please click on the Download QuickTime button below.
* Garcia C, Haque R, Poletti E. Surgical Pearl: Artificial skin model for simulation of flap mechanics. JAAD 2005;53:143-144
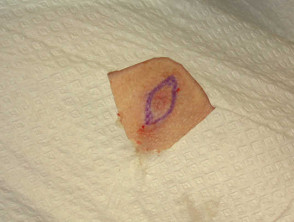
Shave biopsy
Lesions that are entirely above the skin surface can be shaved off as a tangential excision using a No.10 scalpel blade or the flexible DermaBlade®. Light cautery or other method of haemostasis is used and the wound left to heal by secondary intention.
Shave biopsy
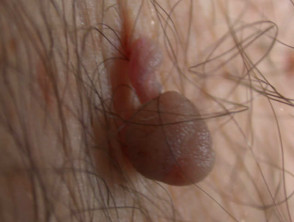
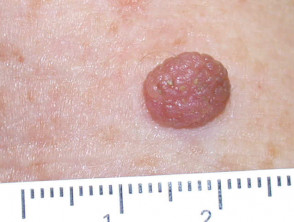
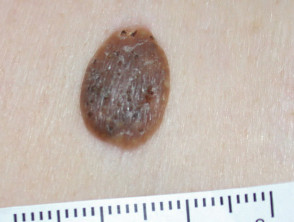
Typical lesions removed this way include:
- Large skin tags
- Filiform viral warts
- Seborrhoeic keratoses
- Papillomatous melanocytic naevi
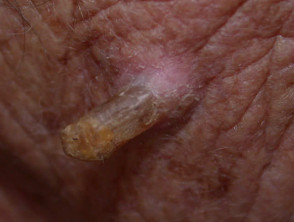
- Cutaneous horn
- Hyperkeratotic actinic keratosis
Lesions suitable for therapeutic shave biopsy
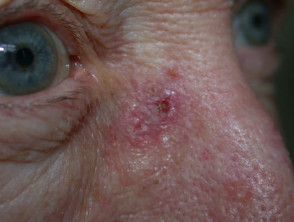
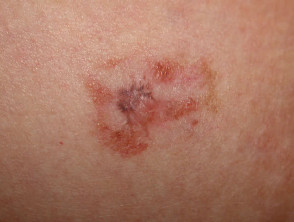
This technique may also be used to sample a flat skin lesion such as a possible basal cell carcinoma or in situ squamous cell carcinoma, with the removal of 3 to 4mm of tissue from the active edge of the lesion. Dermatologists sometimes evaluate suspicious pigmented lesions this way, for example a facial lentigo but this is not recommended in general practice. The specimen should be laid out on a piece of card so it does not curl. Considerable experience is required to obtain an adequate sample for the pathologist.
Lesions suitable for diagnostic shave biopsy
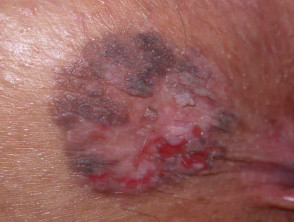
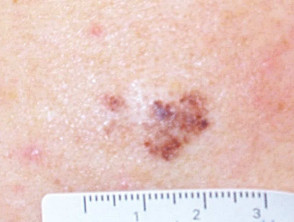
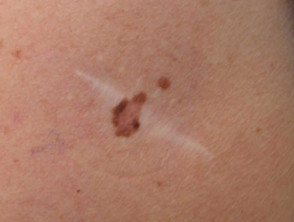
The resultant scar may be unsightly. The lesion may recur, a concern if the original lesion was a non-melanocytic skin cancer. Recurrent melanocytic naevi can look quite bizarre, resembling malignant melanoma.
Recurrent melanocytic lesions after biopsy
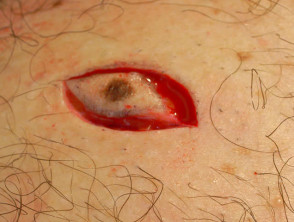
Curettage
Curettage refers to the use of a sharp spoon-like instrument to scrape off a predominantly epidermal superficial skin lesion. The lesion may be shaved off flush with the skin first.
Curettage can be used for the following skin lesions:
- Viral warts (if failed to resolve with salicylic acid)
- Molluscum contagiosum (in adults)
- Seborrhoeic keratoses (thin ones are better frozen)
- Pyogenic granuloma
- Actinic keratoses (most are better frozen)
- In situ squamous cell carcinoma (Bowen disease)
- Keratoacanthoma (if there is no clinical doubt about the diagnosis)
- Superficial basal cell carcinoma (in experienced hands)
- Well demarcated and small nodular or cystic basal cell carcinoma
Lesions suitable for shave/curettage
Curettage is inappropriate for infiltrating or dermal tumours. It should not be used for poorly differentiated squamous cell carcinoma or for melanocytic lesions.
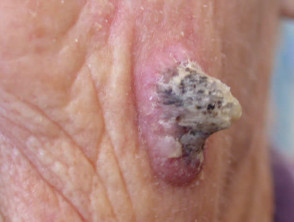
Curettage of malignant skin lesions should be followed by light electrosurgery and repeated to thoroughly remove all the soft tumour cells. Diathermy or cautery may be used for haemostasis. Curettage may be used to de-bulk a larger skin tumour prior to formal excision biopsy of the defect. Occasionally curettage may be followed by cryotherapy or photodynamic therapy.
Shave, curettage and cautery of pyogenic granuloma
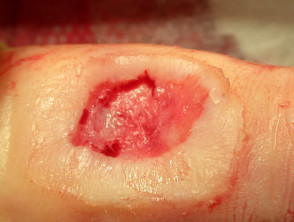
Benign lesions are easier to scrape off if light electrodessication is used first. However, this destroys the tissue and prevents accurate histological examination. Pressure or aluminium chloride may be used for haemostasis after curettage of benign skin lesions to reduce scarring.
Excessive electrosurgery will result in delayed healing and hypertrophic scarring.
Curettage and diathermy
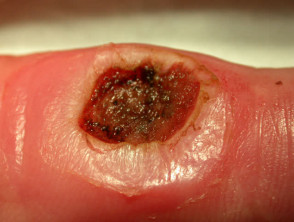
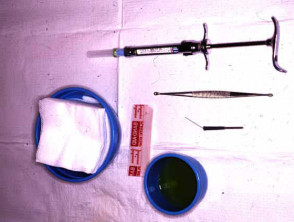
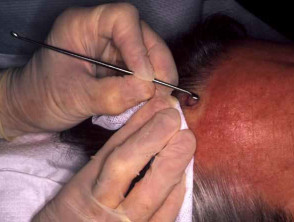
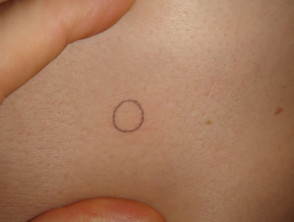
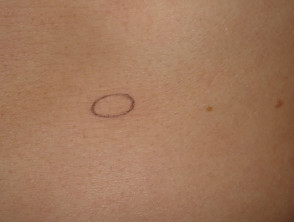
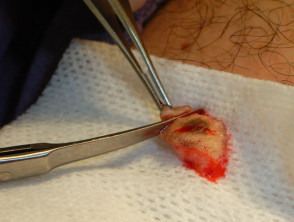
Punch biopsy
Punch biopsy is used to obtain a diagnostic full-thickness skin specimen from a dermatosis or neoplasm. It involves the use of a circular blade or trephine attached to a pencil-like handle. Biopsy punches may have blades of diameter 2 to 7 mm; disposable 3 to 4 mm blades are the most useful. The technique is rather like using a miniature biscuit cutter.
If taking a biopsy of an inflammatory dermatosis, consider whether a pathological diagnosis (which may be non-specific) will change management. If possible, avoid the lower leg and ulcerated, eroded or excoriated lesions. Choose a lesion of recent onset, or the most abnormal-looking edge of an enlarging plaque.
Skin lesions suitable for punch biopsy include suspected squamous cell and basal cell carcinoma. It is inappropriate for suspicious melanocytic lesions; these are better excised as sampling could miss melanoma, as this may arise within a benign lesion.
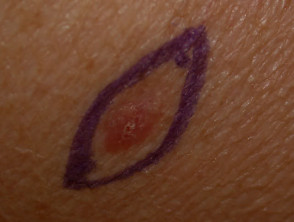
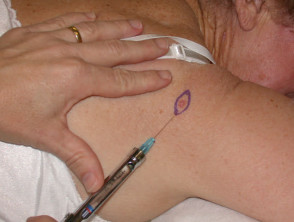
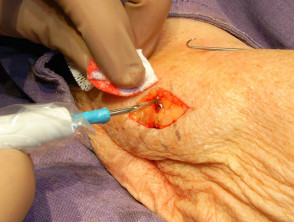
The chosen site should be marked, infiltrated with local anaesthetic (usually 2% lignocaine with adrenaline), cleansed and draped.
Punch biopsy
Stretch the skin perpendicular to the lines of least skin tension so that the eventual wound will be an elliptical shape and under less tension.
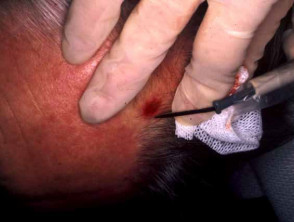
Hold the instrument vertically over the skin. Rotate the biopsy punch down through the epidermis and dermis, and into the subcutaneous fat using a twirling motion, then gently remove it. Use iris scissors to cut the specimen below the dermis. The punch biopsy yields a cylindrical core of tissue that must be gently handled to prevent crush artefact – it is better to extract it with a needle than to use forceps. Place the specimen in the histology pot.
A separate punch biopsy may be taken from perilesional skin for direct immunofluorescence in case of a blistering skin lesions or suspected cutaneous lupus or vasculitis, or from the centre of the lesion for culture in case of suspected atypical mycobacterial or deep fungal infection.
Close the wound(s) using a single 5-0 interrupted monofilament nylon suture (6-0 on the face). Ensure haemostasis by firm pressure then apply a bandage. The suture should be removed 5-10 days later.
Skin lesions that have been surgically removed should always be sent to the pathology laboratory for examination.
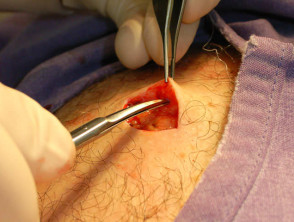
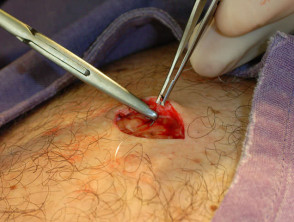
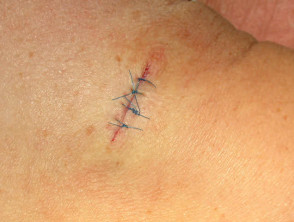
Elliptical excision and closure
Arrange for an experienced practitioner to demonstrate the technique of excision biopsy and supervise your initial efforts. It is easiest to learn on older skin, where the resultant scar will be disguised by skin markings and blemishes. Make sure there is adequate elasticity to allow primary closure of the wound.
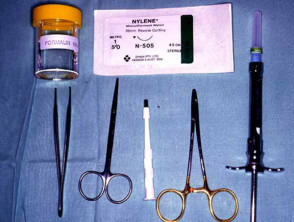
Minimal instruments typically used for excision of a 0.5 to 1 cm diameter skin lesion may include:
- Scalpel e.g. Bard Parker, with #15 blade
- Toothed forceps e.g. Adson
- Iris scissors
- Needle holder
The skin surgery trolley should have been cleaned with hypochlorite and covered with a sterile guard. Equipment should also include:
- Dressing pack
- Antiseptic solution
- Gauze swabs
- Bag for disposal of soiled swabs
- Some form of haemostasis
- Suture, most often monofilament nylon (5-0 face and neck, 4-0 trunk and limbs)
You will also need a pottle containing 10% formol saline for histology, and a container for used sharps.
Getting ready for skin surgery
Identify the margin of the lesion in a good light using magnification. A basal or squamous cell carcinoma should be removed with a 3-4 mm margin as the tumours are generally larger than clinically evident.
Suspected melanoma should be excised with a 2-3 mm margin initially. Narrow margins are recommended for melanocytic lesions for the following reasons:
- Definitive excision margins of confirmed melanoma are selected according to the histological depth of tumour
- Management may include sentinel node biopsy, which requires an intact lymphatic system
- Minimal cost and risk of complications and scarring should the lesion prove benign
Use a surgical pen to mark out a fusiform shape around the lesion. It should be two or three times as long as it is wide, and follow the lines of least tension. On the face, place the excision markings in the line of a wrinkle or parallel to it. In the absence of wrinkles, create dynamic lines by asking the patient to smile, frown or shut their eyes tightly.
Skin surgery
Infiltrate with local anaesthetic, (usually 2% lignocaine with adrenaline) using a short (half- to one-inch) and fine needle (27-30 gauge) injected into the superficial dermis. Generally, 0.5 ml is sufficient to anaesthetise an area of about 1cm2.
Scrub up using standard techniques or waterless antiseptic cleanser approved for surgical use (e.g. 3M™ Avagard™ surgical antiseptic). Don surgical gloves and gown. Cleanse the surgical area with povidone iodine, chlorhexidine or alcohol solution (not adjacent to the eyes). Apply surgical guards for aseptic technique.
With one hand, stabilise the incision area by traction. Using controlled pressure on the scalpel, cut along the marked lines vertically with the angle of the blade slightly oriented away from the lesion. Dissect down to mid subcutaneous tissue, and remove the specimen.
One end of the ellipse may be snipped off as a simple way of orientating the specimen (provide the pathologist with a labelled diagram on the request form). Place the entire lesion in a labelled histology pottle.
If necessary, use blunt-tipped scissors undermine the skin edge by about 5 mm below the dermis to free up the overlying skin, to allow suturing without tension. Obtain haemostasis.
Skin surgery
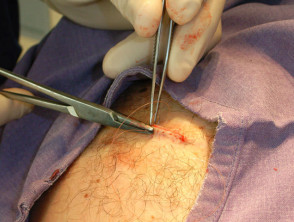
Close the wound. There are several techniques. The simplest is a single layer closure using simple interrupted loop sutures tied using the needle holder, inserting and removing the needle in the line of its curve. There should be an equal ‘bite’ of each side including at the least the whole dermis. Tie the surgical 'reef' knot so the edges of the wound are lightly in contact and slightly everted. Cut the sutures 5-10 mm from the skin surface. In some sites a continuous suture may be satisfactory for small wounds.
Deeper larger wounds may require insertion of interrupted absorbable subcutaneous sutures to eliminate dead space (which enhances the chance of haematoma and infection).
Suturing
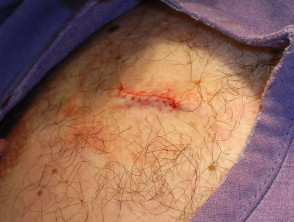
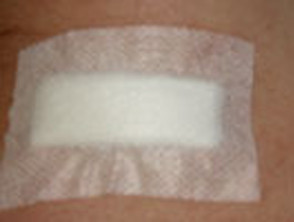
Apply light pressure to ensure bleeding has completely stopped, clean the wound and apply a protective dressing for at least 24 hours. Typically, smear petroleum jelly over the excision line and apply a non-adherent hypoallergenic adhesive dressing. A waterproof dressing may be preferred.
Applying a dressing
Advise the patient regarding wound care. In most cases, the first dressing should be removed at 24 hours and the wound should be gently cleansed with tap water. Dressings are then used if required to protect the wound from injury.
Sutures should be removed very gently as soon as possible (to avoid suture marks), using scissors or a scalpel blade and fine dissecting forceps. The number of days after the procedure depends on body site, the size of the wound and the amount of tension on it:
- Face: 4-7 days
- Arms: 7-10 days
- Trunk: 8-12 days
- Lower legs 12-14 days
Mohs micrographic surgery
The indications for Mohs surgery are:
- Primary non-melanoma skin cancer in high-risk areas such as mid face
- Recurrent or incompletely excised tumours
- Morphoeic basal cell carcinoma.
If a Mohs surgeon is unavailable, these lesions are removed by standard techniques with wide margins.
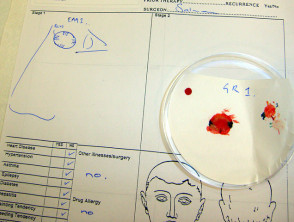
Mohs surgery is performed under local anaesthesia. The majority of the lesion is first removed using a curette (a sharp spoon-shaped instrument). An intact two to three millimetre layer is then taken around the margins and under the base of the tumour. The lesion must be precisely orientated, divided, colour coded and mapped. The frozen specimen is prepared by taking 3mm thick horizontal sections using a microtome, which are then processed and stained. It allows identification of any remaining tumour on the slide, the map and patient so that a second stage of excision can be specifically directed at residual tumour. The process is repeated until the tumour is clear without removing unnecessary unaffected skin.
The wound may be left to heal by secondary intention but is more frequently reconstructed.
Mohs micrographic surgery
Activity
Compare published cure rates for basal cell carcinoma treated by various different methods.
Related information
References
- Bath-Hextall F, Bong J, Perkins W, Williams H.Interventions for basal cell carcinoma of the skin: systematic review. BMJ. 2004 Sep 25;329(7468):705. Epub 2004 Sep 13.
On DermNet NZ
Information for patients
Other websites
- Emedicine Dermatology: Surgical Articles
- SCARD. Skin cancer audit tool for primary care
Books about skin diseases
See the DermNet NZ bookstore.
Sign up to the newsletter
© 2024 DermNet.
DermNet does not provide an online consultation service. If you have any concerns with your skin or its treatment, see a dermatologist for advice.