Introduction
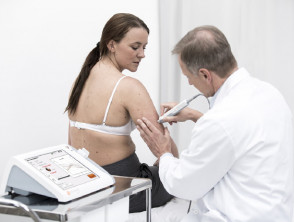
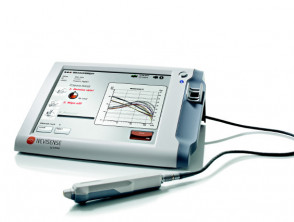
Electrical impedance spectroscopy (EIS) is used by a point-of-care portable device called Nevisense™ (Scibase AB, Stockholm, Sweden) to objectively analyse lesions with suspicion of melanoma.
Nevisense™ electrical impedance spectroscopy*
*Images supplied by Scibase
What is electrical impedance spectroscopy?
Visual examination usually is sufficient when identifying most types of skin lesion. However, when it comes to atypical lesions, a clinical diagnosis based on only a visual examination may pose a challenge, leading to unnecessary excisions or even missed malignancies.
Dermatoscopy uses a hand-held skin surface microscope to examine skin lesions. In expert hands increases the chance of correct diagnosis, but requires training and considerable experience.
In the spectrometric analysis of skin lesions, a computer software program is used that calculates and extracts information about the cells and structures of the skin. The method uses a light beam that penetrates beneath the skin surface. Light images taken with a digital camera or hand-held scanner are fed into a computer for detailed analysis.
In electrical impedance spectroscopy, the varying electrical properties of human tissue are used to categorise cellular structures and thereby detect malignancies.
SciBase Electronic Impedance Spectroscopy is a patented technology developed at the Karolinska Institute in Stockholm, Sweden.
How does electrical impedance spectroscopy work?
- Skin tissues have different electrical properties under different medical conditions.
- Based on this, it is possible to identify a condition, such as melanoma, using Nevisense's unique EIS method.
- Electrical impedance spectroscopy is a measure of the overall resistance within the tissue at alternating currents of various frequencies.
- An electrical signal is applied through the skin lesion using an innovative electrode system on the tip of the Nevisense™ probe.
- The frequencies used by Nevisense™ (1 kHz – 2.5 MHz) relate to clinically relevant properties, such as the composition of intra- and extracellular environments, cell shape and size, and cell membrane composition.
- To cover the lesion in both width and depth, the measurement is performed at 35 frequencies and four depth settings over the lesion in a total of 10 permutations.
- A reference measurement is performed at first, on healthy skin close to the lesion.
- This procedure is repeated on the lesion to be examined.
- Nevisense's advanced algorithm classifies the lesion based on measurement data from both the lesion and the reference.
- The Nevisense™ classifier then provides an EIS score output on the Nevisense™ screen reflecting the degree of atypia identified by the method.
- The results of the visual inspection of suspected melanoma can then be added to objective information provided by Nevisense™ to reach a more informed clinical decision.
What are the advantages of electrical impedance spectroscopy analysis?
Electrical impedance spectroscopy analysis complements the physicians' visual examinations, particularly in cases of cutaneous lesions with unclear clinical signs of melanoma. Benefits include:
- A fast and simple procedure
- Increased diagnostic accuracy
- Objective analysis.
What clinical evidence supports the efficacy of electrical impedance spectroscopy?
- Electrical impedance spectroscopy has undergone evaluation for more than a decade, from development and proof of principle to clinical studies in skin cancer and benign skin lesions.
- In a multi-centre, a prospective and blinded study conducted at 5 US and 17 European investigational sites, a total of 1,951 subjects with 2,416 lesions were enrolled in the study.
- All eligible skin lesions in the study were examined with the Nevisense™ electrical impedance spectroscopy system and by excisional biopsy subjected to histopathological evaluation.
- 1,943 lesions were evaluable for the primary efficacy endpoint (including 265 melanomas, 48 basal cell carcinomas and seven squamous cell carcinomas).
- Results of the pivotal study showed 97% sensitivity for malignant melanoma and specificity of 34.4%.
- The observed sensitivity for nonmelanoma skin cancer was 100%.
- The positive and negative predictive values of Nevisense™ were 21.1% and 98.2%, respectively.
- The observed sensitivity and specificity for melanoma of the investigational site’s histopathologists were 85.0% and 98.1%, respectively.
- No serious adverse events or unanticipated adverse device effects were observed throughout the study.
- However, there is insufficient data to determine the role of Navisense™ in the management of patients with melanoma.
What is the clinical use of electrical impedance spectroscopy?
- The Nevisense™ EIS system is intended for use on cutaneous lesions with one or more clinical or historical characteristics of melanoma.
- The system is designed to be used when a clinician chooses to obtain additional information when considering excision.
- It is not meant to be used to confirm a clinical diagnosis of melanoma.
- It should be used by physicians trained in the clinical diagnosis of skin cancer.
What are the indications for electrical impedance spectroscopy?
- Electrical impedance spectroscopy is indicated for use on primary skin lesions with a diameter between 2 mm and 20 mm.
- Lesions must have intact skin (ie, non-ulcerated and non-bleeding lesions).
- Lesions should not be in hair-covered areas.
- The device should not be used on palms, soles, genitalia, eyes or mucosal areas.
- Lesions should be devoid of scars or recent trauma.