Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Author: Dr Beth Wright, Dermatology Registrar, Bristol, United Kingdom, August 2016.
Introduction - alpha-1-antitrypsin deficiency disease Introduction - panniculitis Demographics Causes Clinical features Diagnosis Treatment General measures Oral medications Outcome
Alpha-1-antitrypsin is a serine protease inhibitor. Alpha-1-antitrypsin deficiency disease is an inherited metabolic disorder in which this protein is absent or defective.
Alpha-1-antitrypsin deficiency disease most commonly presents as lung disease (emphysema) or gastrointestinal disease (liver cirrhosis). It can also rarely affect the skin.
Alpha-1-antitrypsin deficiency is abbreviated as A1AT deficiency.
Panniculitis is an inflammation of the subcutaneous adipose tissue (the fat underneath the skin).
Panniculitis due to alpha-1-antitrypsin deficiency is rare.
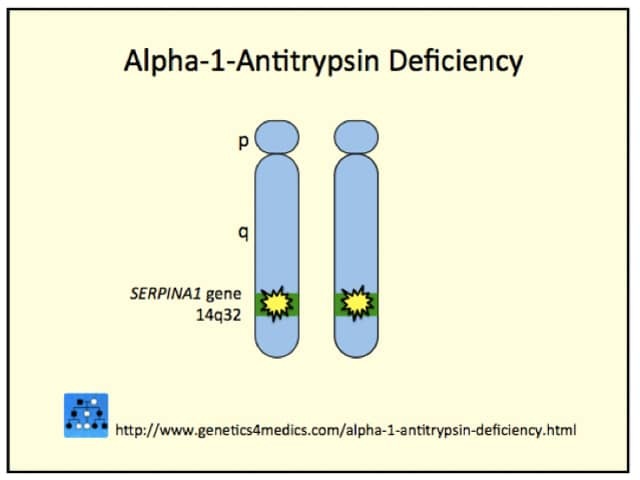
Genetics of alpha-1-antitrypsin deficiency*
*Image courtesy Genetics 4 Medics
Alpha-1-antitrypsin deficiency is most prevalent in Caucasians of European and North American descent.
Males and females of any age are equally affected by the panniculitis associated with alpha-1-antitrypsin deficiency, and it can rarely arise in children.
As alpha-1-antitrypsin deficiency is under-diagnosed, the true prevalence of panniculitis due to alpha-1-antitrypsin deficiency is difficult to ascertain. One study reported panniculitis affected 0.9% of ZZ homozygotes for alpha-1-antitrypsin deficiency.
Panniculitis may be preceded by trauma. This is because an injury causes increased protease activation and a cascade of inflammatory events intended to heal the wound.
Alpha-1-antitrypsin deficiency is usually due to a mutation on chromosome 14.
Alpha-1-antitrypsin deficiency is an autosomal co-dominant disorder, meaning that two different versions (alleles) of a gene are expressed in an individual. Each allele makes a slightly different protein. Both alleles influence the characteristics and severity of the deficiency disease.
There are over 90 known mutant alleles of the alpha-1-antitrypsin gene, and they are classified based on their acid starch gel mobility (F=fast, M=medium, S = slow, Z = very slow). A normal level of a variant enzyme with altered activity can also cause clinical disease.
The genotype (a person’s genetic makeup) and their combination of these genes determine the degree of the deficiency. The normal genotype is ‘MM’ has a normal level of alpha-1-antitrypsin. Patients with two ‘ZZ’ genes (homozygotes) are 85% deficient in alpha-1-antitrypsin. This type is the most commonly associated with emphysema.
Alpha-1-antitrypsin is mostly produced in the liver and regulates several proteolytic enzymes including trypsin, elastase, chymotrypsin, factor VIII, collagenase and kallikrein. Lack of alpha-1-antitrypsin leads to increased activity of these enzymes and localised tissue destruction.
Deficiency of alpha-1-antitrypsin leads to inflammation and necrosis in various organs.
In alpha-1-antitrypsin deficiency disease:
The panniculitis associated with alpha-1-antitrypsin deficiency disease does not improve with antibiotics or systemic steroids.
Alpha-1-antitrypsin deficiency disease may be suspected by the clinical features, especially emphysema, chronic hepatitis, and liquefying panniculitis. Several tests may be arranged to confirm the diagnosis.
Serum alpha-1-antitrypsin levels
Alpha-1-antitrypsin phenotype and genotyping
Respiratory investigations
Blood tests
Skin biopsy
A deep incisional skin biopsy is required for panniculitis to include deep adipose tissue. Specific characteristics of alpha-1-antitrypsin deficiency panniculitis pathology include:
Systemic corticosteroids, antimalarials, immunosuppressants and danazol are ineffective in alpha-1-antitrypsin deficiency disease.
Enzyme replacement therapy with exogenous alpha-1-antitrypsin concentrate (Prolastin®) infused intravenously has been reported to be effective. The initial recommended dose of 60 mg/kg once weekly may be adjusted according to response. The dose required and duration of treatment varies according to case reports. Its use may be limited by high cost and limited availability.
The prognosis and life expectancy of a patient with alpha-1-antitrypsin deficiency depend on phenotype, the level of enzyme deficiency, and the associated hepatic and pulmonary damage.
Complete resolution of panniculitis associated with alpha-1-antitrypsin deficiency has been described within four weeks of treatment with enzyme replacement therapy. It may recur, in which case indefinite treatment may be required.
Chronic ulceration can complicate the associated panniculitis, and when areas do finally heal they may form atrophic scarring.