What is drug hypersensitivity syndrome?
Drug hypersensitivity syndrome is a specific, severe, unexpected reaction to a medicine, which affects several organ systems at the same time. It typically causes a combination of:
- High fever
- Morbilliform eruption
- Haematological abnormalities
- Lymphadenopathy
- Inflammation of one or more internal organs.
Drug hypersensitivity syndrome is sometimes also called drug reaction with eosinophilia and systemic symptoms (DRESS), and drug-induced hypersensitivity syndrome (DIHS).
The syndrome is classified as a severe cutaneous adverse reaction (SCAR). It may have overlapping features with Stevens–Johnson syndrome / toxic epidermal necrolysis (SJS/TEN) and acute generalised exanthematous pustulosis (AGEP).
Who gets drug hypersensitivity syndrome?
Drug hypersensitivity syndrome is relatively rare. It mainly affects adults and is equal in incidence in males and females. Genetic susceptibility and HLA associations have been found for several causative drugs.
The most common drugs to cause this reaction are a number of anticonvulsant drugs (particularly carbamazepine, phenobarbital, and phenytoin), the anti-gout drug, allopurinol, olanzapine, and the sulphonamide group of antibiotics. It has been estimated that at least 1 in every 10,000 patients treated with an anticonvulsant will develop drug hypersensitivity syndrome.
The risk of drug hypersensitivity syndrome in patients on allopurinol depends on the dose of allopurinol, associated kidney disease, and concomitant thiazide diuretics.
It has been rarely reported with many other medications. It can be very difficult to determine the exact cause of drug hypersensitivity syndrome if several medicines have been commenced in preceding weeks. In about 10% of cases, the causative drug is never identified.
Drug hypersensitivity syndrome occurring within 2 weeks of starting the responsible drug is most likely with beta-lactam antibiotics or iodinated contrast media. onset is delayed more than 2 weeks with anticonvulsants and allopurinol.
What causes drug hypersensitivity syndrome?
Drug hypersensitivity syndrome is a delayed T cell-mediated reaction. Tissue damage is due to cytotoxic T cells and cytokine release.
- There is a genetic predisposition to drug hypersensitivity syndrome.
- A defect in the way the liver metabolises drugs may be responsible.
- Re-activation of human herpesvirus 6 (HHV6, the cause of roseola) or Epstein-Barr virus (EBV) may also be important.
What are the clinical features of drug hypersensitivity syndrome?
Drug hypersensitivity syndrome usually develops over several days, with onset 2-8 weeks after starting the responsible medicine.
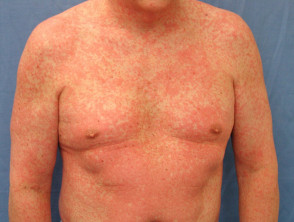
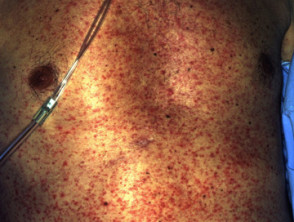
A high fever of 38–40 C is usually noticed first. This is quickly followed by a widespread skin rash. Characteristics of the rash are diverse.
- Morbilliform eruption affects 80% of cases, with varying morphology including targetoid lesions, blisters and pustules
- Erythroderma or exfoliative dermatitis (involving > 90% body surface area) may follow in about 10%
- Facial swelling affects 30%
- Mucosal involvement affects 25% (lips, mouth, throat, genitalia)
The rash can last many weeks.
Drug hypersensitivity syndrome rash
Systemic involvement in drug hypersensitivity syndrome
Symptoms may worsen after stopping the drug and may continue for weeks, or even months, despite drug withdrawal. The severity of the rash does not necessarily correlate with the extent of internal organ involvement. Later symptoms depend on the internal organs affected. They may include:
- Enlarged lymph nodes in several sites (75%)
- Haematological disorders: raised white count (or less often, reduced white count), eosinophilia (in 30% this is > 2.0 x 109/L), atypical lymphocytes, thrombocytopenia, anaemia, haemophagocytic syndrome
- Liver enlargement, hepatitis, and rarely hepatic necrosis with liver failure. Abnormal liver function tests are found in 70–90%.
- Kidneys are affected in about 10%, and is usually a mild interstitial nephritis. Renal failure is rare.
- Inflammation of the heart (myocarditis) or pericarditis causing chest pain, breathlessness, and lowered blood pressure
- Lung involvement causes shortness of breath and cough (interstitial pneumonitis, pleuritis, pneumonia and acute respiratory distress syndrome)
- Neurological involvement may lead to meningitis and encephalitis, polyneuritis, causing headache, seizures, coma, and palsies
- Gastrointestinal symptoms: gastroenteritis, pancreatitis, bleeding and dehydration. In severe cases, acute colitis and pancreatitis can occur, and chronic enteropathy may follow.
- Endocrine abnormalities: thyroiditis and diabetes
- Myositis
- Uveitis.
What are the complications of drug hypersensitivity syndrome?
Causes of death from drug hypersensitivity syndrome include:
- Acute liver failure causing coagulation problems, jaundice, and impaired consciousness
- Multiorgan failure
- Fulminant myocarditis
- Haemophagocytosis.
How is drug hypersensitivity syndrome diagnosed?
The diagnosis of drug hypersensitivity syndrome is based on the clinical triad of:
- High fever
- Extensive skin rash
- Organ involvement.
It is supported by eosinophilia and abnormal liver function tests.
As drug hypersensitivity syndrome can occur up to eight weeks after first exposure to the responsible drug, a great degree of care is required when determining the responsible medication. A temporal association between medicine use and the start of the syndrome is the strongest evidence.
The European Registry of Severe Cutaneous Adverse Reactions to Drugs and Collection of Biological Samples (RegiSCAR) has produced diagnostic criteria to assist in the diagnosis of drug hypersensitivity syndrome. RegiSCAR inclusion criteria for potential cases require at least 3 of the following:
- Hospitalisation
- Reaction suspected to be drug-related
- Acute skin rash
- Fever above 38C
- Enlarged lymph nodes at two sites
- Involvement of at least one internal organ
- Blood count abnormalities such as low platelets, raised eosinophils, or abnormal lymphocyte count.
Attempts to confirm which drug has caused drug hypersensitivity syndrome may include patch tests. Patch testing has been reported to be most successful for anticonvulsant drugs, with 50% positive reactions. It is not useful for allopurinol, with 0% positive reactions. Lymphocyte transformation testing is available in some centres, where specialist interpretation may reveal the causative drug in the majority of cases.
Investigations in drug hypersensitivity syndrome
After taking a careful history and performing skin and general examination, the following tests may be requested.
Skin biopsy usually shows dense infiltration of inflammatory cells, including lymphocytes and eosinophils, extravasated erythrocytes, and oedema.
Blood tests may include:
- Blood count and coagulation studies
- Biochemical tests: at least liver function, renal function, muscle enzymes
- Viral serology: hepatitis B, C, EBV, CMV, HHV-6
- Endocrine function: thyroid, glucose levels
- Genetic susceptibility tests.
Urinalysis is undertaken to assess renal damage.
Cardiac and pulmonary evaluation may include electrocardiograph (ECG), echocardiogram, and chest X-ray. Scans may be performed to evaluate the liver, kidneys, and brain depending on clinical findings and the results of initial tests.
What is the treatment for drug hypersensitivity syndrome?
Treatment consists of immediate withdrawal of all suspect medicines, followed by careful monitoring and supportive care. It is very important for patients presenting with a high fever and a rash, where a diagnosis of drug hypersensitivity syndrome is considered, to have blood tests as soon as possible.
Systemic corticosteroids (eg, prednisone) are generally used in the more severe cases of drug hypersensitivity syndrome with significant exfoliative dermatitis, pneumonitis, and/or hepatitis. However, the benefits of corticosteroids are unknown as controlled clinical trials are lacking. Once effective, they should be withdrawn very slowly as the syndrome can recur as the dose reduces.
Ciclosporin has been reported to be an alternative and effective treatment for drug hypersensitivity syndrome.
Additional treatment may include intravenous immunoglobulin, plasmapheresis, and immunomodulatory drugs such as cyclophosphamide, mycophenolate, and rituximab.
Supportive treatment for the skin rash may include:
- Dressings
- Topical steroids
- Emollients
- Oral antihistamines.
Fluid, electrolytes, and calorie intake may need attention. A warm environment and expert nursing care are required. Secondary infections may require antibiotics.
How can drug hypersensitivity syndrome be prevented?
Patients who develop drug hypersensitivity syndrome must avoid taking the causative medicine(s) again.
Cross-reactions are common between the three main aromatic anticonvulsant drugs (phenytoin, carbamazepine, phenobarbitone). Patients who have experienced drug hypersensitivity syndrome with any one of these medicines must avoid all three.
Because genetic factors are suspected in drug hypersensitivity syndrome, first-degree relatives should be alerted to their elevated risk of developing hypersensitivity reactions to the same medicine(s).
What is the outlook for drug hypersensitivity syndrome?
Most patients fully recover from drug hypersensitivity within weeks to months. Relapse after initial improvement is common.
Patients recovering from drug hypersensitivity syndrome are thought to be at risk of developing autoimmune diseases.
The mortality from drug hypersensitivity syndrome is estimated at around 8%.