What is an intralesional steroid injection?
An intralesional steroid injection involves a corticosteroid such as triamcinolone acetonide injected directly into a lesion on or immediately below the skin.
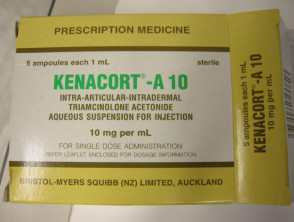
In New Zealand, triamcinolone injection is marketed as Kenacort-A and is available in 2 strengths: 10 mg per ml (Kenacort-A 10) and 40 mg per ml (Kenacort-A 40). Triamcinolone acetonide is marketed as Kenalog in the USA. Betamethasone injection is marketed as Celestone Chronodose (1 mL) and is not available in New Zealand.
Shorter-acting corticosteroid preparations, such as dexamethasone or betamethasone acetate, are sometimes administered in combination with triamcinolone.
What are intralesional steroids used for?
An intralesional steroid injection may be indicated for the following skin conditions:
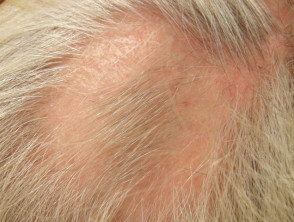
- Alopecia areata
- Discoid lupus erythematosus
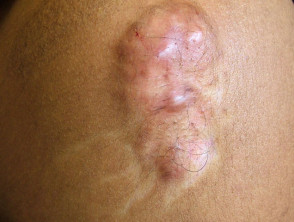
- Keloid/hypertrophic scar
- Granuloma annulare
- Other granulomatous disorders such as cutaneous sarcoidosis or granuloma faciale
- Hypertrophic lichen planus
- Lichen simplex chronicus (neurodermatitis)
- Localised psoriasis
- Necrobiosis lipoidica
- Acne cysts (see nodulocystic acne) and inflamed epidermoid cysts
- Small infantile haemangiomas
- Other localised inflammatory skin diseases.
Hair regrowth after intralesional steroid into alopecia areata
What are the benefits of intralesional steroids?
Intralesional administration of corticosteroids treats a dermal inflammatory process directly. In contrast to topical steroids, intralesional steroids:
- Bypass the barrier of a thickened stratum corneum
- Reduce the chance of epidermal atrophy (surface skin thinning)
- Deliver higher concentrations to the site of the pathology.
Other uses for triamcinolone acetonide injection
Triamcinolone injection is also sometimes used intramuscularly (rather than as an intralesional injection) systemically as an alternative to oral corticosteroids, for example for seasonal hay fever, or to treat a chronic skin disorder such as atopic dermatitis or lichen planus.
Typical intramuscular doses are 0.5–1 mg/kg body weight (40–80 mg for a typical adult), which may be repeated every 30 days for 3–6 months.
Triamcinolone injections can also be used in the treatment of tendonitis, arthritis, and synovitis.
What are the contraindications to intralesional steroid injection?
Intralesional steroids should not be injected at the site of active skin infection (eg, impetigo or herpes simplex).
They must not be used if there is a known triamcinolone allergy.
When large doses of triamcinolone acetonide are used as an alternative to oral steroids such as prednisone, they are considered to be systemic steroids. These should be avoided in patients with the following disorders:
- Active tuberculosis or systemic fungal infection
- Extensive plaque psoriasis, pustular psoriasis or erythrodermic psoriasis — systemic steroids may destabilise psoriasis
- Active peptic ulcer disease
- Uncontrolled diabetes, heart failure or severe hypertension
- Severe depression or psychosis
How is intralesional steroid administered?
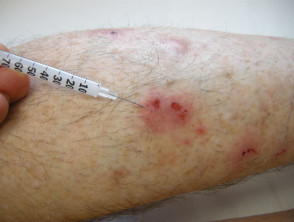
Intralesional triamcinolone is injected directly into the skin lesion using a fine needle after cleaning the site of injection with alcohol or antiseptic solution. The injection should be intradermal, not subcutaneous, to avoid causing a dent in the skin.
The initial dose per injection site will vary depending on the lesion being treated. Generally, 0.1–0.2 mL is injected per square centimetre of involved skin. The total dose should not normally exceed 1–2 mL per dose. It can be repeated every 4–8 weeks.
The corticosteroid can be full strength (eg, triamcinolone 10 mg/mL or 40 mg/mL) or diluted with normal saline or local anaesthetic. Typical regimes for triamcinolone intralesional injections include:
- 40 mg/mL for a thick keloid scar
- 10 mg/mL for a moderate thickness hypertrophic scar
- 10 mg/ml into discoid lupus erythematosus or granuloma annulare
- 5 mg/ml into the skin of normal thickness associated with alopecia areata.
The injections may be repeated monthly for a few months while the lesions are active.
Intralesional steroid injection
What side effects may arise at the site of an intralesional steroid injection?
Side effects and risks of intralesional triamcinolone may be separated into early and delayed effects.
Early effects tend to be self-limited. They include:
- Pain, bleeding, bruising
- Infection
- Contact allergic dermatitis due to the preservative, benzyl alcohol
- Impaired wound healing
- Sterile abscess, sometimes requiring surgical drainage.
Delayed adverse effects include:
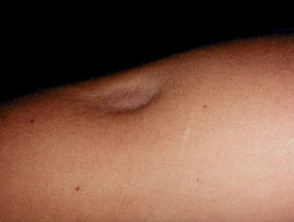
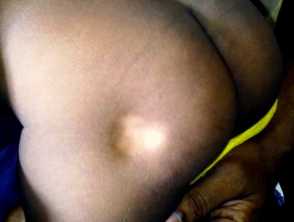
- Cutaneous and subcutaneous lipoatrophy (most common) — skin indentations or dimples around the injection sites a few weeks after treatment; these may be permanent
- White marks (leukoderma) or brown marks (postinflammatory pigmentation) at the site of injection or spreading from the site of injection; these may resolve or persist long term
- Telangiectasia at the site of injection, which can be treated if necessary by laser or intense pulsed light (IPL).
- Localised hypertrichosis; this resolves eventually.
- Localised or distant steroid acne: steroids increase growth hormone, leading to increased sebum production by the sebaceous glands. Steroid acne generally improves once the steroid has been stopped.
Side effects of intralesional steroid injection
Systemic side effects of triamcinolone injections
Allergic reactions are very rare and are dose-independent. They may include local or generalised urticaria (wheal and flare), and in more severe cases, anaphylaxis.
Other systemic side-effects are not likely to follow the intralesional injection of localised skin disease because the dose used is very small.
The following potentially serious conditions have been reported from intramuscular injection of large doses of triamcinolone acetonide.
- Heart: congestive heart failure in susceptible patients, fluid retention, hypertension, cardiac arrhythmias.
- Hormonal effects: decreased glucose tolerance, Cushing syndrome, hirsutism, hypertrichosis, diabetes mellitus, menstrual irregularities, adrenocortical and pituitary unresponsiveness, and the suppression of growth in children.
- Musculoskeletal disease: aseptic necrosis of hip or shoulder bones, calcinosis, osteoporosis and pathological fractures, muscle weakness, and tendon rupture.
- Neurologic/psychiatric symptoms: convulsions, depression, euphoria, swelling of the brain, insomnia, and mood swings.
- Eyes: glaucoma, cataracts, and rare instances of blindness associated with periocular injections.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).