What is Merkel cell carcinoma of the skin?
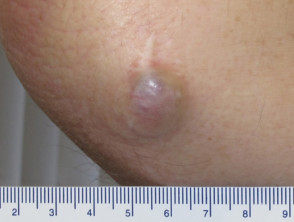
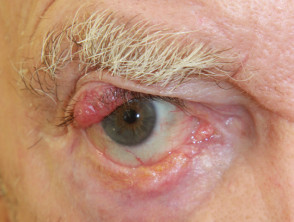
Merkel cell carcinoma of the skin is a rare form of skin cancer. It may be very aggressive and often metastasises to other parts of the body. It has also been called Toker tumour, cutaneous neuroendocrine carcinoma, trabecular cell carcinoma, and primary small-cell carcinoma of the skin.
Merkel cell carcinoma
Who gets Merkel cell carcinoma?
Merkel cell carcinoma has an estimated incidence of 0.23 per 100,000 people in Caucasian populations, which is much less common than melanoma.
- Increasing numbers of Merkel cell carcinomas have been reported by some centres in recent years.
- Merkel cell carcinoma mainly affects older people, with most cases occurring after the age of 50.
- It is slightly more common in men.
- It occurs on parts of the body commonly exposed to sunlight, most often the head and neck.
- It is also more common and more serious in those that are immune suppressed, such as patients with solid organ transplants, human immunodeficiency virus (HIV) infection, haematological malignancy or on drugs such as azathioprine.
What are the clinical features of Merkel cell carcinoma?
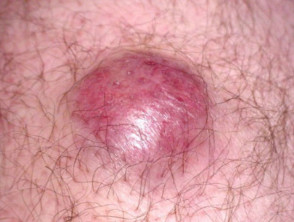
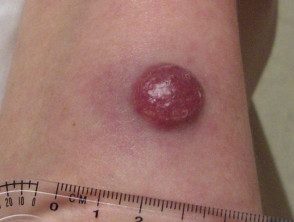
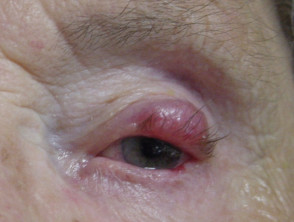
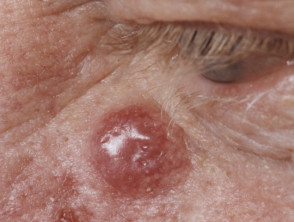
Merkel cell carcinoma usually presents as a rapidly enlarging, solitary, irregular red nodule. It is often similar in appearance to other more common skin cancers such as basal cell carcinoma but grows much more quickly.
Merkel cell cancers spread through the lymphatic system and multiple metastases can develop around the main tumour (local recurrence). Merkel cell carcinoma may also spread to lymph nodes in the neck, axillae and groin. This is more likely in thicker tumours. Most recurrences occur within the first two years after diagnosis.
Merkel cell carcinoma
What causes Merkel cell carcinoma?
Merkel cell polyomavirus (MCPyV) has been detected in about 80% Merkel cell carcinomas tested. It is thought that the virus causes gene mutations leading to Merkel cell carcinoma when immune function is defective. The virus-negative tumours are associated with high exposure to ultraviolet radiation, due to the occurrence of the tumour on sun-exposed skin.
Immunosuppression is an important factor for the development of Merkel cell carcinomas.
Merkel cell carcinoma was previously believed to arise from Merkel cells, which are pressure receptors in the skin. A recent investigation is pointing to their origin being early B-cells (lymphocytes) based on cellular morphology, the expression of early B-cell markers and clonal immunoglobulin chain rearrangement.
How is Merkel cell carcinoma diagnosed?
Merkel cell carcinoma should be considered in any tumour with "AEIOU" clinical features, which are present in about 90% of patients with the disease.
- Asymptomatic or non-tender
- Expanding rapidly
- Immune suppressed
- Older than 50
- UV-exposed fair skin
The main test is a biopsy of the tumour. This shows characteristic Merkel cell carcinoma pathology. Immunohistochemistry can be helpful as cytokeratin-20 (CK20) is positive in up to 95% of tumours and thyroid transcription factor (TTF1) is usually negative.
A general examination, including evaluation of local lymph nodes, and staging investigations may be arranged to determine whether the tumour has spread to other sites.
Staging investigations may include:
- Sentinel node biopsy
- Lymph node ultrasound scan
- Imaging using X-rays, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) scans.
A specific Merkel cell carcinoma staging system is published by the American Joint Committee on Cancer.
Longterm survival is likely if the lymph nodes do not contain tumour cells.
What is the treatment of Merkel cell carcinoma?
Once Merkel cell carcinoma is diagnosed, multidisciplinary consultation is usual. As 5-year survival rates for MCC are only around 50%, early aggressive treatment is required, most often with a combination of surgery and radiation therapy.
Localised disease
Surgical excision is the main treatment of primary Merkel cell carcinoma. A wide area surrounding the tumour may also be removed; this may not be necessary if radiotherapy is added.
The primary site may be treated with radiotherapy postoperatively, especially for large lesions (> 2 cm). Radiation treatment leads to increased local and regional disease control and higher long-term survival rates.
The relevant lymph nodes may also be surgically removed or irradiated as a prophylactic measure.
Disease involving regional lymph nodes
If cancer has spread to involve the lymph nodes, these may be surgically removed or treated with radiotherapy. In some cases, systemic chemotherapy may also be administered.
Distant metastatic disease
Any distant metastatic Merkel cell carcinoma is very serious and has a very poor prognosis. Treatment of the metastatic disease is aimed at improving quality of life. In some cases, radiotherapy and/or systemic chemotherapy may be administered for treatment.
A positive response has been reported in up to 50% of patients with advanced Merkel cell carcinoma when treated with the immune checkpoint inhibitors pembrolizumab and nivolumab. Response to PD-1 blockade can occur in virus-positive and virus-negative subtypes. The response appears to be less impressive in patients who had previously received chemotherapy. Clinical trials are on-going. Note that new Merkel cell carcinoma has been reported during checkpoint inhibitor treatment of other forms of cancer.
In March 2017, the US the Food and Drug Administration granted accelerated approval to another PD-1/PD-L1 agent, avelumab (Bavencio®) to treat metastatic Merkel cell carcinoma. Approval was based on a trial of avelumab that showed a response in one-third of patients.
Experimental treatment with other agents is being explored.
Consensus treatment guidelines are published by the National Comprehensive Cancer Network.
What is the outcome for patients with Merkel cell carcinoma?
Five-year survival rates are overall 30–50%. Survival rates are better for patients initially diagnosed with Stage 1 Merkel cell carcinoma and in younger patients than when it has already metastasised or patients are over 80 years of age.