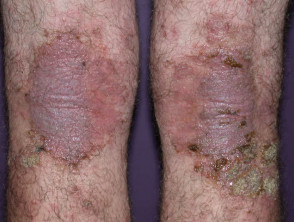
What is upadacitinib?
Upadacitinib (RINVOQ®) is an oral, second-generation selective Janus kinase (JAK) inhibitor used for atopic dermatitis (eczema). It targets the JAK1 enzyme and suppresses the immune response.
The US Food and Drug Administration (FDA) approved upadacitinib for use in January 2022 for the treatment of moderate to severe atopic dermatitis.
More images of atopic dermatitis
What is upadacitinib used for?
In dermatology, upadacitinib is used for adults and adolescents (12 years and older) with moderate to severe atopic dermatitis who are candidates for systemic therapy.
- It can be used as monotherapy or in combination with topical steroids.
- Clinical trials showed ≥75% improvement in the Eczema Area and Severity Index (EASI) score from baseline to week 16.
Other uses:
- Rheumatoid arthritis
- Psoriatic arthritis
- Ankylosing spondylitis
- Non-radiographic axial spondyloarthritis
- Ulcerative colitis.
What are the contraindications and precautions with upadacitinib?
Contraindications
- Concurrent use of other immunomodulating agents such as other Janus kinase (JAK) inhibitors, biologics, or potent immunosuppressants (eg, azathioprine, cyclosporine, tacrolimus).
- Hypersensitivity to upadacitinib or its excipients.
- Pregnancy (category D) and breastfeeding:
- Upadacitinib was found to be teratogenic and present in breastmilk in animal studies; there is no human evidence to date.
- Severe hepatic impairment (Child-Pugh C).
- Active and serious infections (eg, tuberculosis, hepatitis B, hepatitis C).
Precautions
- Patients with risk factors for thrombosis (eg, past history of thromboembolism, obesity, prolonged immobilisation).
- Elderly patients (age >65).
- Paediatric populations.
- Severe renal impairment (CrCl <30mL/min).
- Co-administration with other CYP3A4 inhibitors (eg, ketoconazole, itraconazole, clarithromycin) or CYP3A4 inducers (eg, rifampicin, phenytoin).
- Consider the risk and benefits of starting upadacitinib in patients with known malignancies.
- Ensure effective contraception during treatment and for at least 4 weeks after cessation.
Consider the need for immunisations prior to commencing upadacitinib. Live vaccinations should not be given during or just before starting treatment. Live vaccines include measles-mumps-rubella (MMR), typhoid, bacillus Calmette-Guerin (BCG), yellow fever, varicella zoster, rotavirus, and Japanese encephalitis.
Dosing
Upadacitinib is available as 15 mg modified-release oral tablets. The dosing for the treatment of atopic dermatitis is 15 mg once daily. If response is inadequate after 4 weeks, consider increasing to 30 mg once daily.
If elderly (age >65), or severe renal impairment (CrCl <30mL/min), the maximum dose is 15 mg once daily. For paediatric patients aged ≥12 years and weighing ≥40 kg, dosing is 15 mg once daily, increased to 30 mg if response is inadequate.
Monitoring
Prior to commencing upadacitinib, investigations include:
- Complete/full blood count
- Kidney function
- Liver function tests
- Lipid studies
- Tuberculosis screening with IRGA test and chest X-ray, hepatitis B, and hepatitis C screening
- Pregnancy test if relevant.
Monitoring during treatment with upadacitinib includes routine full blood count, liver enzymes, and lipid studies (12 weeks after treatment). Ensure routine skin examinations due to increased risk of non-melanoma skin cancers.
Treatment should be ceased if:
- Haemoglobin <80 g/L
- Absolute neutrophil count (ANC) <1 x 109 cells/L, lymphocytes <0.5 x 109 cells/L
- Signs of active infection (eg, reactivation of herpes zoster) — increased risk of serious and opportunistic infections while on treatment.
What are the benefits of upadacitinib?
- Oral formulation, once daily dosing.
- Rapid onset of action.
- High level of skin clearance, itch improvement, and improved quality of life in patients with moderate to severe atopic dermatitis.
What are the disadvantages of upadacitinib?
- Need to consider multiple drug interactions.
- Careful monitoring with routine blood tests required during treatment.
- Risk of serious infections.
- Not safe in pregnancy or breastfeeding.
- Immunisations with live vaccines should not be given during or just before starting treatment. See also: Immunisation in immunosuppressed dermatology patients.
What are the side effects and risks of upadacitinib?
Upadacitinib is generally well tolerated with a favourable benefit-risk profile.
Common side effects:
- Upper respiratory tract infections (eg, sinusitis, pharyngitis, tonsillitis)
- Nausea
- Fever
- Cough
- Headache
- Abdominal pain
- Weight gain
- Hypercholesterolaemia
- Acne
- Folliculitis.
Uncommon side effects:
- Anaemia
- Neutropenia
- Lymphopenia
- Increased liver enzymes
- Increased creatinine kinase
- Serious and opportunistic infections
- Viral reactivation (eg, herpes zoster, hepatitis)
- Thrombosis: deep venous thrombosis, pulmonary embolism, or arterial thrombosis.
Rare side effects:
- Gastrointestinal perforation
- Malignancy (data in clinical trials are limited; ongoing long-term studies):
- Increased risk of lymphoma in patients with rheumatoid arthritis
- Non-melanoma skin cancers observed in patients treated with upadacitinib.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).