What is guselkumab?
Guselkumab is a biological treatment indicated for moderate-to-severe psoriasis.
The US Food and Drug Administration (FDA) has approved guselkumab (Tremfya™, Janssen Biotech, PA, USA) for the treatment of adults living with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Guselkumab is the first and only approved biological therapy that selectively blocks only interleukin 23 (IL-23), a cytokine that plays a key role in plaque psoriasis.
Applications seeking approval of guselkumab in the European Union, Japan, and other countries are currently under review.
In July 2020, guselkumab was approved by the FDA for the treatment of psoriatic arthritis.
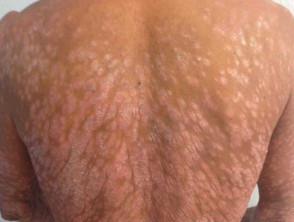
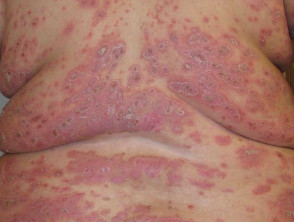
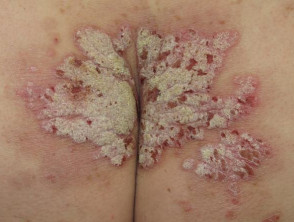
Severe plaque psoriasis
What is guselkumab used for?
- Guselkumab is used for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy (ultraviolet radiation treatment).
- It is not known if guselkumab is safe and effective in children under 18 years of age.
How does guselkumab work?
- Guselkumab is a human monoclonal immunoglobulin G1 lambda (IgG1λ) antibody that selectively binds to the p19 subunit of IL-23 (a protein involved in the inflammatory response) and inhibits its interaction with the IL-23 receptor.
- While IL-23 promotes normal inflammatory and immune responses, the p19 and p40 subunits of IL-23 are found to be over-expressed in the condition of psoriasis and other autoimmune inflammatory skin diseases.
- Guselkumab selectively binds to the p19 subunit of IL-23 in dendritic cells and keratinocytes and blocks its interaction with the IL-23 receptor, which further prevents the release of other pro-inflammatory cytokines and chemokines via stimulation of immune cells, such as T helper 17 (Th17) cells.
- Guselkumab thus blocks the abnormally heightened signalling of inflammatory cascades (the chain reaction process of activating the immune response) that promote epidermal abnormalities, including keratinocyte hyperproliferation (unsuppressed wound healing) and psoriatic plaque formation.
How is guselkumab given?
- Guselkumab is administered by subcutaneous injection.
- The recommended dose is 100 mg at Week 0, Week 4, and every 8 weeks thereafter.
- Guselkumab should not be injected into areas where the skin is tender, bruised, red, hard, thick, scaly, or affected by psoriasis.
- It is intended for use under the guidance and supervision of a physician.
- Guselkumab may be administered by a health care professional, or a patient may self-inject after proper training in subcutaneous injection technique.
- Guselkumab may increase the risk of infection.
- A missed dose should be injected as soon as it is remembered, and the next dose taken at the appropriate scheduled time.
Pre-treatment evaluation for tuberculosis and immunisation
- Treatment with guselkumab should not be started in patients with any clinically important active infection until the infection resolves or is adequately treated.
- Patients with a history of latent tuberculosis should be suitably treated prior to administration of guselkumab, because it can reactivate latent TB infection.
- Patients should not be immunised with live vaccines during treatment with guselkumab.
Use in specific populations
Pregnant women
- There are no data are available for the use of guselkumab in pregnant women, indicating there is a drug-associated risk related to major birth defects and miscarriage.
- No adverse developmental effects in offspring from 0–6 months of age were observed in cynomolgus monkeys treated with guselkumab at up to 30 times the maximum recommended dose for humans.
- See the DermNet page on the safety of medicines taken during pregnancy.
Breastfeeding mothers and infants
- There is no information on the presence of guselkumab in human milk or its effects on the breastfed infant.
- The risk–benefit potential should be considered when prescribing guselkumab to the mother.
- See the DermNet page on lactation and medications used in dermatology.
Children
The safety and effectiveness of guselkumab in paediatric patients < 18 years of age have not been evaluated.
Older people
Clinical studies with guselkumab did not include sufficient numbers of patients aged 65 years to determine whether they respond differently from younger subjects.
Patients with hepatic or renal impairment
No trials have been conducted to assess the effect of hepatic or renal impairment on the pharmacokinetics of guselkumab.
Adverse effects of guselkumab
The most common adverse reactions (≥ 1% of patients) reported include:
- Upper respiratory infections
- Headache
- Injection site reactions
- Arthralgia
- Diarrhoea
- Gastroenteritis
- Tinea infection
- Herpes simplex infection.
Drug interactions with guselkumab
- There are no data are available on the ability of live or inactive vaccines to elicit an immune response in patients receiving treatment with guselkumab.
- Live vaccines should not be used concomitantly during guselkumab treatment.
- Population pharmacokinetic analyses have indicated that the concomitant use of ibuprofen, aspirin, or paracetamol does not affect the clearance of guselkumab.
- The effect of drugs metabolised via the hepatic cytochrome P450 enzymes (eg, warfarin and ciclosporin) may be altered during concomitant administration with guselkumab. Dosage modification of these drugs should be considered.
- Results from an exploratory drug–drug interaction study in patients with moderate-to-severe psoriasis have suggested a low potential for clinically relevant drug interactions for drugs metabolised by CYP3A4 (midazolam), CYP2C9 (warfarin), CYP2C19 (omeprazole), and CYP1A2 (caffeine), but the interaction potential cannot be ruled out for drugs metabolised by CYP2D6 (metoprolol).
Contraindications to guselkumab
Guselkumab should not be used in patients that:
- Have an infection that does not go away or that keeps coming back
- Have or have been in close contact with someone with TB
- Recently received or are scheduled to receive a vaccine
- Plan to become pregnant
- Breastfeeding or plan to breastfeed.
Guselkumab is promising for plaque psoriasis in Phase III studies.
- Guselkumab has shown an acceptable safety profile and good efficacy in the treatment of moderate-to-severe plaque psoriasis.
- It is a first-in-class biological therapy that selectively blocks only IL-23, a cytokine that plays a key role in plaque psoriasis.
- Guselkumab has demonstrated superior results in skin clearance compared with adalimumab in head-to-head analyses.
- Clinical trial findings have also demonstrated the effectiveness of guselkumab in patients who had an inadequate response to treatment with ustekinumab.
- A Phase III study evaluating guselkumab in the treatment of active psoriatic arthritis is ongoing, and a Phase III programme evaluating the efficacy of guselkumab compared with secukinumab in the treatment of moderate-to-severe plaque psoriasis is under way.
- Although further studies are needed to assess long-term safety and efficacy, based on the results to date, guselkumab appears to be a promising therapeutic option for moderate-to-severe plaque psoriasis.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).