Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Treatments Autoimmune/autoinflammatory
Author: Vanessa Ngan, Staff Writer, 2006. Updated by Chief Editor Hon Assoc Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand, February 2015.
Introduction Efficacy How it works How to use Tests prior to adalimumab Vaccinations and adalimumab Precautions Adverse effects Monitoring Interrupting adalimumab
Adalimumab belongs to the class of biologic medicines called tumour necrosis factor (TNF) inhibitors. It is approved in adults and children over 4 years of age for the treatment of psoriasis, psoriatic arthritis, polyarticular juvenile idiopathic arthritis, ankylosing spondylitis, rheumatoid arthritis, ulcerative colitis and Crohn disease, and uveitis. In September 2015, the FDA also approved adalimumab for the treatment of hidradenitis suppurativa. In New Zealand (October 2019), PHARMAC has approved funding on Special Authority application in certain circumstances for severe treatment-resistant psoriasis, moderate to severe hidradenitis suppurativa, Behcet syndrome and pyoderma gangrenosum.
The original brand of adalimumab has the trade name Humira®. Generic biosimilar adalimumab is also marketed in several countries.
Adalimumab has been studied in several thousand adult patients with moderate to severe psoriasis. The efficacy of the medication was judged using PASI (Psoriasis Area and Severity Index) scores before treatment and after using adalimumab 40 mg every 2 weeks. More than 70% of patients achieved a 75% reduction in the PASI score at 16 weeks, and in 15–20%, psoriasis cleared up completely.
Adalimumab is not a cure for psoriasis and must be continued long term. Although it continues to be effective in the majority of patients, sometimes psoriasis recurs despite on-going adalimumab injections. This is called secondary failure.
Adalimumab 40 mg every 2 weeks has also been studied in several hundred patients with psoriatic arthritis. Most patients get some improvement in joint pain and swelling in at least a few affected joints and some patients report remarkable benefit from the treatment.

Psoriasis before commencing adalimumab
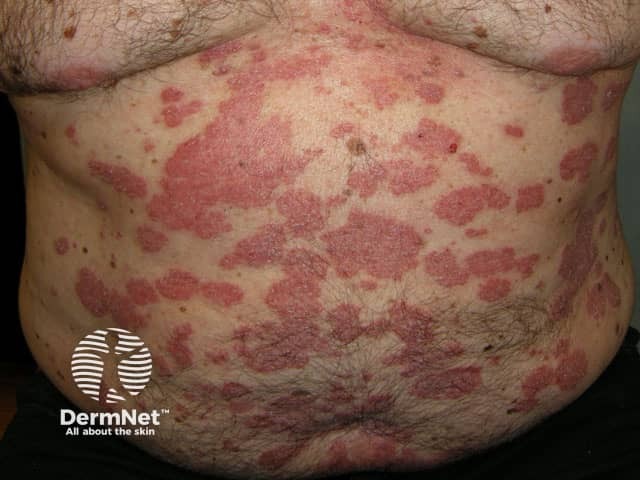
Psoriasis before commencing adalimumab

Psoriasis before commencing adalimumab

Psoriasis 6 months after commencing adalimumab
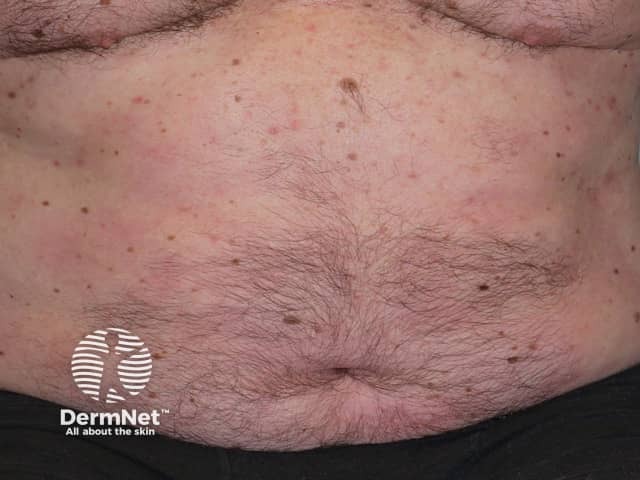
Psoriasis 6 months after commencing adalimumab

Psoriasis 6 months after commencing adalimumab
See more images of psoriasis treated with adalimumab.
Adalimumab is a recombinant monoclonal antibody that contains only human peptides. It works by directly binding to TNF molecules in the blood and diseased tissue. TNF bound to adalimumab is prevented from causing the inflammation that results in psoriasis plaques.
Adalimumab is also effective for other inflammatory skin diseases; it is registered for the treatment of hidradenitis suppurativa. In New Zealand, it is funded to treat some cases of Behçet disease and pyoderma gangrenosum.
Adalimumab is administered by subcutaneous injection once every two weeks. It is available as a pre-prepared syringe or pen. After initial counselling and training, patients can usually self-inject into the thigh or abdomen. A different site should be used at each injection to reduce soreness and prevent the skin from becoming tender, red, bruised or hard.

Removing the caps from the pen

Giving the subcutaneous injection

Pressing on the injection site
The recommended dose for treating psoriasis is 80 mg then 40 mg a week later, followed by 40 mg every fortnight as a single dose.
Other medications including methotrexate and acitretin can usually be continued during treatment with adalimumab.
Dermatologists are likely to request routine blood count, liver and renal function, fasting lipids and antinuclear antibody (ANA) before starting adalimumab. In females, a pregnancy test may be appropriate.
Patients should be screened for tuberculosis (TB), active or latent viral hepatitis (HAV, HBV, HCV) and human immunodeficiency virus (HIV). If present, these infections should be treated before starting adalimumab.
Immunisation status should be reviewed prior to starting adalimumab. If necessary, vaccines should be updated prior to treatment. Annual influenza vaccination is recommended.
As they may induce illness in immunodeficient individuals, live vaccines should not be used during treatment with adalimumab. Currently, the available live attenuated viral vaccines include measles, mumps, rubella, varicella, yellow fever, influenza (intranasal vaccine) and the oral polio vaccine. Live attenuated bacterial vaccines include BCG and oral typhoid vaccine.
Read more about immunisation in immunosuppressed dermatology patients.
Because adalimumab works by selectively targeting TNF, theoretically it should not have much effect on the rest of the body's immune system. However, caution must be taken when considering its use in patients prone to infections or in those with active chronic or recurrent infections. Concurrent treatment with corticosteroids, azathioprine or ciclosporin increases the risk of infection. Infection can be due to bacterial, mycobacterial, invasive fungal (disseminated or extrapulmonary histoplasmosis, aspergillosis, coccidioidomycosis) viral, parasitic, or other opportunistic infectious agents.
There is a particular concern that etanercept may reactivate tuberculosis (TB) (including risk of Bacillus Calmette-Guérin [BCG]), viral hepatitis B, C, and increase the risk of the human immunodeficiency virus (HIV), listeria and legionella infections.
Immunisation with live vaccines (such as yellow fever, varicella, zoster, mumps/measles/rubella [MMR], BCG) must be avoided.
Adalimumab should also be used with caution in the following situations:
Patients on anakinra, a drug used in rheumatoid arthritis, should not be prescribed adalimumab.
The safety of adalimumab during pregnancy and breastfeeding is unknown. It is therefore not recommended. Where possible, the drug should be discontinued several months before conception.
Patients who require major surgery may be advised to stop adalimumab temporarily 2–3 months prior to a planned operation. It can be started again 2 weeks after surgery providing no infection is present.
Adalimumab appears to be well tolerated. Mild to moderate injection site reactions (redness, swelling, itching, pain) appear to be the most common side effect, occurring in 20% of patients. Mild to serious infections are the main risk of treatment and should be promptly treated.
Severe cutaneous reactions have rarely been reported, including worsening psoriasis, vasculitis, anaphylaxis, Stevens–Johnson syndrome and toxic epidermal necrolysis. Adalimumab can cause drug-induced vitiligo.
Like all medications that work on the immune system, it may increase the risk of certain types of lymphoma (white blood cell cancer). These have rarely been reported in patients on adalimumab, usually in those also taking other medicines that suppress the immune system such as azathioprine or mercaptopurine.
Skin cancers, in particular, squamous cell carcinoma, have also been reported in patients on adalimumab, usually in patients with other risk factors such as sun-damaged skin or previous treatment with photochemotherapy (PUVA).
Regular follow-up visits to monitor the safety and efficacy of treatment are necessary. It is recommended that patients on biologic medications have routine blood tests every 6 months or so, including full blood count and liver function tests. TB testing should also be repeated from time to time.
Blood levels of adalimumab may be used to monitor adherence to therapy and determine the optimum dose in an individual.
Adalimumab should be discontinued in the following circumstances:
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).