Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Treatments Lesions (cancerous)
Author: Steven Lamb MBChB, Dermatology Registrar, Waikato Hospital, Hamilton, New Zealand, 2000. Updated by Dr Darion Rowan, Dermatologist, Middlemore Hospital, Auckland, New Zealand; Hon A/Prof Amanda Oakley, Dermatologist, Hamilton, New Zealand, 2004.
Introduction
Uses
How it works
Treatment regimes
How to use
Results
Side effects
Imiquimod is an immune response modifier. It is manufactured as a 5% (50 mg/g) cream called Aldara™. Generic forms of imiquimod cream are also available. The Perrigo brand is funded by PHARMAC in New Zealand (2019).
A 3.75% cream called Zyclara™ has also been approved by the FDA in the USA but is not yet available in New Zealand (March 2017).
Imiquimod is mainly used to treat:
It was first approved for use in genital warts. It may also be helpful in the unapproved treatment of common warts (once they have been thinned down by other means), plane warts, and herpes simplex. Experimentally it has been successful in reducing some keloid scars, granuloma annulare, vitiligo, melanoma in situ (lentigo maligna type) and, in combination with diphencyprone, metastatic melanoma (see topical and intralesional immunotherapy for melanoma.
Although sometimes prescribed for molluscum contagiosum, clinical trials have not shown imiquimod to be of benefit, and it use for this indication is not recommended.
Before starting, a biopsy may be performed to confirm the diagnosis. Imiquimod is particularly useful for field changes, superficial skin cancers with poorly defined margins, and in areas where surgery or other treatments may be difficult, complicated or otherwise undesirable, especially the face and lower legs.
Imiquimod works by stimulating the immune system to release a number of chemicals called cytokines, which are important in fighting viruses and destroying cancer cells.
When used to treat skin cancers and pre-cancerous lesions it results in inflammation, which destroys the lesion. The degree of inflammation is quite variable from person to person, in part due to the type of skin lesion and in part due to genetic factors. Imiquimod is taken up by ‘toll-like receptor 7’ on specific immune cells found in the epidermis; these receptors are expressed more in some individuals and in some skin lesions than in others.
Various imiquimod treatment regimes are used. For example:
The imiquimod should be applied less often if the reaction is excessive, and more often if no reaction occurs (perhaps accompanied by a topical retinoid to enhance absorption of imiquimod into the skin). The course can be repeated, and treatment is sometimes continued for up to 16 weeks.
Treatment should be carefully monitored, because the cream may need to be applied more or less frequently than originally planned or for a shorter or longer course, depending on response. Once the inflammation has settled there is generally a good or excellent cosmetic result with little scarring.
The 5% product is supplied either as a box of sachets, each containing 250 mg, or as a pump-pack (2 g). Wash hands before and after applying imiquimod cream.
Cut the top off the sachet or pierce the sachet with a needle and squeeze out a tiny amount of cream onto your fingertip. If using the pump pack, remove the protective cap and prime several times before pressing the pump once only to dispense the cream. Apply the cream to the affected areas.
Although the information on the packet states that the sachet is for single-use, some people seal it using a paper clip or tape and store it in a closed container to prevent the cream drying out.
Imiquimod is usually used during the winter months but, as it is not photosensitising, it may be used at any time of year. Sun protection with clothing and sunscreen can continue as usual.
Imiquimod in the genital area should be washed off before having sexual intercourse. It can weaken rubber condoms.
Areas treated with imiquimod will become inflamed. The effects include itching, burning, redness, ulceration (sores), scabbing, flaking, and pain. These reactions indicate that the cream is likely to be effective - if there is no inflammation, imiquimod is unlikely to clear the lesion. An exaggerated response may clear the skin lesion sooner than expected — sometimes after as few as three or four applications. In some patients, surrounding untreated areas also become inflamed but this will settle when treatment is discontinued.
If black scabs and ulceration occur or there are severe systemic symptoms, the cream should be discontinued.
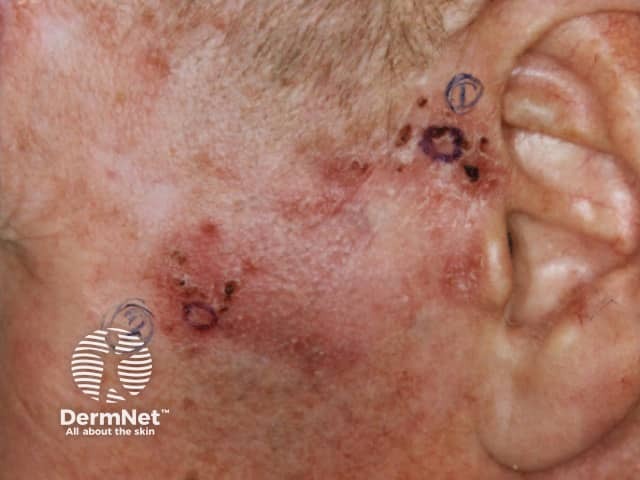
BCC
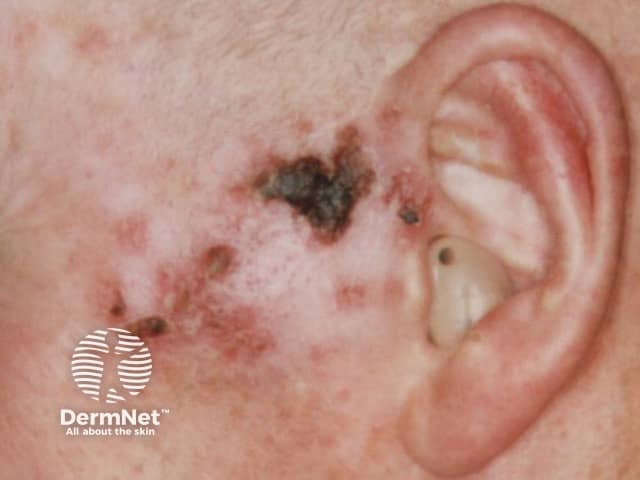
Imiquimod to BCC
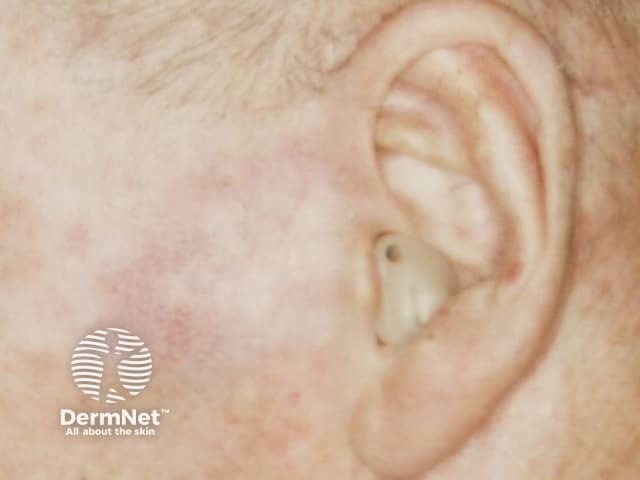
Imiquimod to BCC
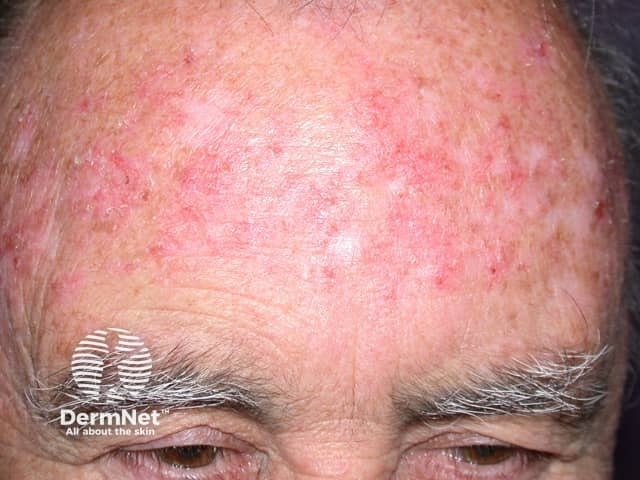
Imiquimod to actinic keratosis
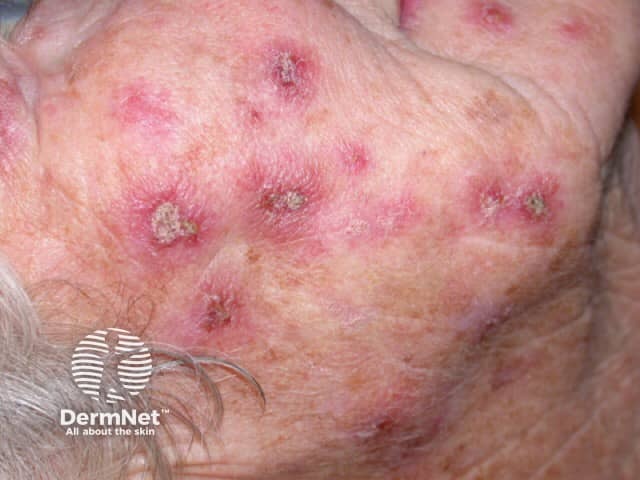
Imiquimod to actinic keratosis
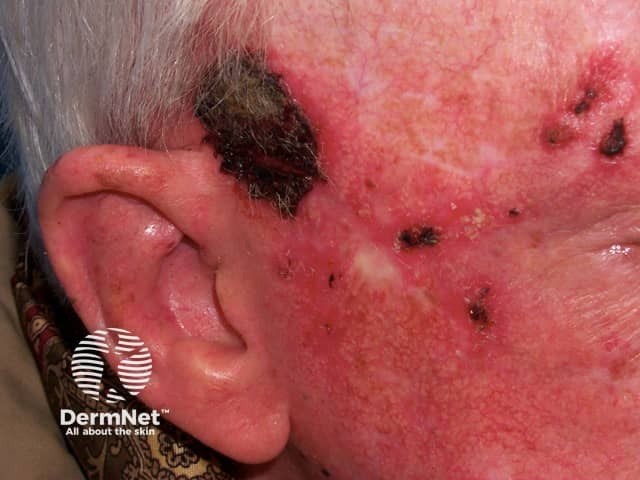
Imiquimod to actinic keratosis
More images of the effect of imiquimod.
When using imiquimod cream, 'flu-like symptoms may develop, such as fever, fatigue, headache, nausea, diarrhoea and muscle pain. These are generally mild and may be treated with paracetamol. Side effects should resolve within a few days of stopping treatment. They may also resolve with continuing treatment.
Imiquimod has been reported to cause drug-induced pemphigus, reactivate pre-existing pemphigus in remission as well as worsen other autoimmune diseases including rheumatoid arthritis. The use of topical imiquimod therefore in patients with a pre-existing autoimmune condition should be undertaken with caution.
Severe inflammation induced by imiquimod can result in drug-induced vitiligo.
Approved datasheets are the official source of information for medicines, including approved uses, doses, and safety information. Check the individual datasheet in your country for information about medicines.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).