Main menu
Common skin conditions
NEWS
Join DermNet PRO
Read more
Quick links
Author(s): Dr Hannah Ross, Dermatology Registrar; and Dr Tsui Ling, Consultant Dermatologist, Photobiology Unit, Salford Royal Hospital, Manchester, United Kingdom (2023)
Previous contributors: Dr Amanda Oakley, Dermatologist (1997)
Reviewing dermatologist: Dr Ian Coulson (2023)
Edited by the DermNet content department
Introduction
Photopatch tests
Phototests
Photoprovocation tests
Laboratory investigations
Phototesting comprises several investigations that are undertaken to determine if a patient has a skin condition caused or aggravated by ultraviolet (UV) exposure.
It can be useful in confirming or excluding photosensitivity, defining the action spectrum of a photosensitive eruption, and diagnosing sunscreen allergies.
Full phototesting consists of:
Not all of the above tests may be indicated for each patient.
Patients should take care to follow the instructions from their photodermatology clinic, including if/when any medications or topical agents should be discontinued prior to testing. For example, steroid creams and antihistamines are usually stopped at least 48 hours before phototesting, as these can affect results.
Monochromator light testing is an essential aspect of phototesting. The light source used is usually a 2500 watt xenon arc lamp. These lamps have an output that mimics sunlight.
The monochromator is a precision optical device designed to fractionate the light to allow exposure to a clearly defined waveband.
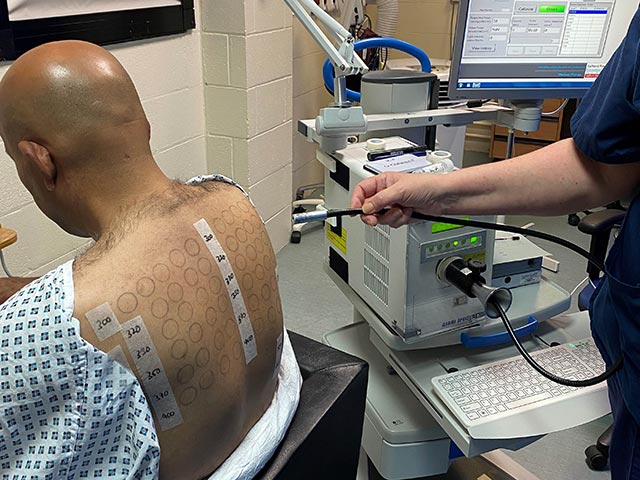
Undertaking monochromator light testing - differing doses of different wavelenghts of UVB, UVA, and visible light are delivered
Photoprovocation can induce an eruption in patients with photosensitivity that have normal erythemal thresholds. It can be useful in the diagnosis of various photosensitive disorders including polymorphic light eruption (PMLE), photoaggravated atopic dermatitis, and actinic prurigo.
The morphology of the artificially-provoked rash assists in diagnosis. A skin biopsy may be performed (with immunofluorescence if indicated) to further confirm the diagnosis. A negative test may not exclude these conditions.
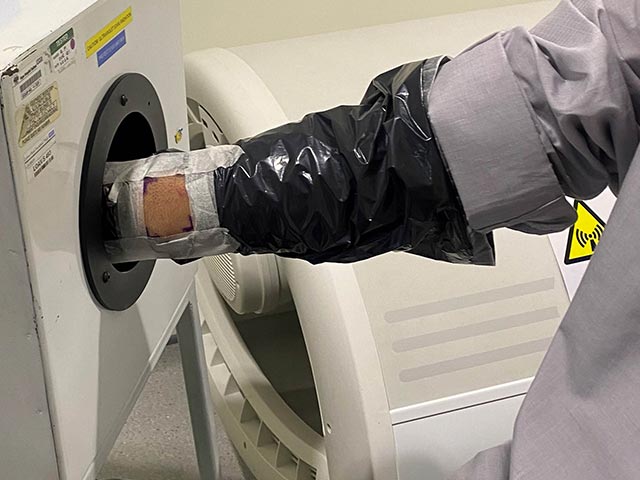
Ultraviolet radiation provocation testing
Photopatch testing aids the diagnosis of suspected contact and/or photocontact sunscreen allergies that may be contributing to photosensitivity. It may also help to guide sunscreen choice for the patient.
As most of the relevant contact allergens are activated by UVA rather than UVB, UVA irradiation is used in photopatch testing. Photopatch test series may include sunscreens, chemical UV filters, and the patient’s own products. Nonsteroidal anti-inflammatory drugs (NSAIDs) are also commonly tested as they can be implicated in photoallergic reactions.
A positive photoallergic response is characterised by a positive response on the irradiated series, with a negative response to the same allergen on the non-irradiated side. A positive response on both sides indicates a contact allergy alone. If there is a stronger response on the UV-irradiated side, this would suggest both contact and photocontact allergy.
If multiple sunscreen allergies are evident, it is advisable to refer on for full patch testing to investigate for coexisting contact allergies.
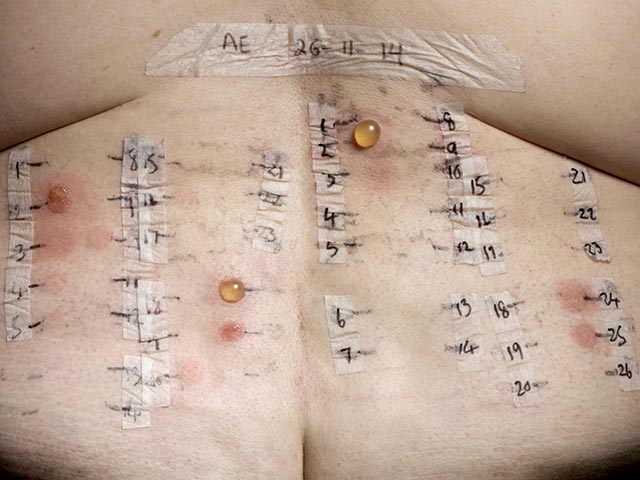
Photocontact testing - two panels of allergens are applied, one set are irradiated with UVA
Laboratory investigations can help formulate a diagnosis in patients with photosensitivity.
Investigations may include:
Phototesting is painless, but skin redness and rashes may be induced during the testing process. Contact dermatitis reactions can be itchy.
Phototesting in isolation seldom provides the diagnosis, except where the tests demonstrate classical solar urticaria or chronic actinic dermatitis.
A diagnosis is often made after correlating symptoms with phototesting findings. However, the diagnosis may not always be certain, due to limitations in patient history, lack of images of skin changes, or equivocal test results.
Following detailed history and examination, phototesting usually concludes the investigations and in most cases is followed by a working diagnosis and management plan.
Patient counselling following phototesting includes:
The range and diversity of the photodermatoses mean that counselling requirements differ. For example, with PMLE a simple explanation of the disorder and its management may suffice. For chronic actinic dermatitis, a more detailed explanation is usually required, particularly in older patients, who may find it challenging to accept the nature of the condition and the need for meticulous photoprotection.
In children with photodermatoses, the counselling is given to the parents, usually with the child in attendance. For severe congenital photosensitivity syndromes such as xeroderma pigmentosum or congenital erythropoietic protoporphyria, this is best managed by a multidisciplinary team. This ensures sufficient expertise to provide the family with a clear understanding of the disorder and its implications, the management plan, and the prognosis.
Some patients may initially be unaccepting of their diagnosis, and appropriate support and encouragement may be needed.
Further appointments may be needed with the referring doctor or the photodermatology clinic if diagnosis or management is not straightforward.