What is rituximab?
Rituximab is a biologic medicine used primarily to treat B-cell lymphoma. Recently, it is has also shown to be useful in the treatment of several severe skin diseases. In 2018, the FDA approved its use for moderate to severe pemphigus vulgaris, an immunobullous disease of the skin.
Rituximab is a monoclonal antibody directed against the CD20 antigen on the surface of normal and malignant B lymphocytes. Rituximab has human and mouse-derived components. Given by intravenous injection, it is very effective at destroying normal and malignant B lymphocytes that are carrying the CD20 antigen. However, it can have severe side effects.
Trade names include Rituxan® and MabThera®.
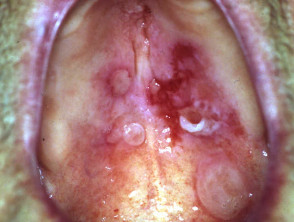
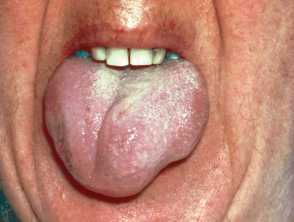
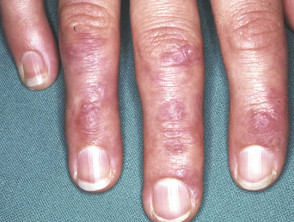
Severe pemphigus vulgaris; rituximab is indicated Severe oral cicatricial pemphigoid unresponsive to steroids and dapsone; rituximab is indicated. Angioedema due to acquired C1esterase deficiency and an underlying lymphoma; it responded to rituximab Severe dermatomyositis of the hands unresponsive to steroids and methotrexate; rituximab is one of several therapeutic options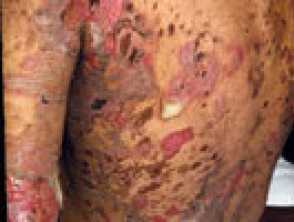
How does rituximab work?
B lymphocytes are a type of white blood cell. Their role in immune reactions includes:
- Production of antibodies, including autoantibodies directed against ‘self’ antigens
- Antigen presentation or identification of a potential protein to attack
- Regulation of T-cell activation
- Production of proinflammatory cytokines.
Rituximab is an immunoglobulin G1 (IgG1) kappa monoclonal antibody composed of a murine (mouse) variable region (Fab portion) that is fused onto a human constant region (Fc portion). The Fab portion binds to the CD20 antigen on the surface of pre-B and mature B lymphocytes. The Fc portion then recruits immune cells that destroy these lymphocytes. Mechanisms may include:
- Complement-dependent cytotoxicity
- Antibody-dependent cell-mediated cytotoxicity
- Apoptosis.
The exact contribution of each mechanism remains unclear, and different mechanisms may prevail in various diseases.
Rituximab has a limited effect on other immune cells.
- The CD20 antigen is not found on stem cells or early pro-B cells so these can regenerate the B lymphocyte population.
- Plasma cells are B cells that do not usually express the CD20 antigen, so they mostly survive. As these produce antibodies, serum immunoglobulin levels tend not to fall dramatically.
- Rituximab may also act on autoreactive T-effector cells, regulatory T cells, and monocyte-derived macrophages.
What is rituximab used for?
Rituximab is indicated for the following conditions:
- CD20 positive B cell non-Hodgkin lymphoma in combination with chemotherapy
- Chronic lymphocytic leukaemia in combination with chemotherapy
- Severe rheumatoid arthritis (RA) in conjunction with methotrexate
- Granulomatosis with polyangiitis and microscopic polyangiitis in combination with systemic steroids
- Moderate-to-severe pemphigus vulgaris.
Rituximab has also been found to be useful in a variety of other immune-mediated and autoimmune disorders in which traditional therapy has failed or resulted in side effects.
It has been reported to be helpful (off-label) for:
- Systemic lupus erythematosus (SLE)
- Nephrotic syndrome
- Antibody-mediated renal transplant rejection
- ABO-incompatible renal transplant
- Other forms of lymphoma and leukaemia
To date, rituximab has been effective in some cases of the following skin conditions:
- Cutaneous B-cell lymphoma
- Primary blistering diseases
- Dermatomyositis
- Graft versus host disease
- Cutaneous vasculitis
- Atopic dermatitis
- Cutaneous lupus erythematosus
- Eosinophilic fasciitis
- Acquired C1-esterase inhibitor deficiency
The most suitable dose of rituximab for these disorders has not yet been determined.
Cutaneous B cell lymphoma
Cutaneous B-cell lymphoma includes follicle centre, marginal zone, and diffuse large B-cell lymphoma, which have all been reported to respond to intravenous rituximab. Direct injections into the skin lesions have also been successful, allowing a lower dose to be used. However, after the initial response, the lymphoma may recur within several months. Retreatment may or may not prove successful as lymphomas can lose CD20 expression and, with that, susceptibility to rituximab.
Primary blistering diseases
Primary blistering diseases (also called autoimmune bullous disorders) are associated with autoantibodies directed against various structural support proteins in the epidermis and dermoepidermal junction.
Rituximab has been successfully used for pemphigus vulgaris and treatment-resistant cases of:
- Pemphigus foliaceus
- Paraneoplastic pemphigus associated with CD20+ lymphoma
- Bullous pemphigoid
- Epidermolysis bullosa acquisita
- Mucous membrane pemphigoid.
Dermatomyositis
Dermatomyositis is an autoimmune disease characterised by inflamed, weakened muscles associated with a characteristic rash. How this occurs is unknown, but it involves T cells, B cells, and antibodies directed against the endothelial cells lining blood vessels in the muscles.
Treatment of treatment-resistant dermatomyositis with rituximab has led to the improvement of muscle and skin disease in several patients. Clinical trials have been set up to determine the role of rituximab in dermatomyositis.
Graft-versus-host disease
Chronic graft versus host disease (GVHD) affects 60–70% of long-term survivors after bone marrow transplantation. It results in lichenoid and sclerodermatous skin changes as well as disease of internal organs. GVHD often fails to respond to conventional treatment, and a quarter of affected patients die from it.
Chronic GVHD has been thought to be due to donor T cells, but drugs targeting T cells have not proved very useful. There is now mounting evidence that B cells are responsible. There have been reports of improvement with rituximab in some patients with GVHD.
Cutaneous vasculitis
Vasculitis is classified as a type-III hypersensitivity reaction involving immune complexes, that is, antibodies bound to antigens in the affected blood vessels. Exactly how rituximab works in vasculitis is unknown.
In April 2011, the US FDA approved rituximab for antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (granulomatosis with polyangiitis and microscopic polyangiitis). Case reports suggest rituximab is also efficacious in cutaneous vasculitis due to cryoglobulinaemia.
Atopic dermatitis
Atopic dermatitis (eczema) results from a complex interaction between various immune cells and proteins and in many patients is characterised by high levels of serum IgE. B cells and plasma cells produce IgE, so theoretically, B-cell depletion could help treat the disease.
Rituximab has resulted in an impressive improvement of atopic dermatitis in some patients with severe disease. However, although researchers confirmed a reduction in circulating B cells in their patients, they found little change in IgE levels.
Cutaneous lupus erythematosus
Rituximab has been successfully used to treat refractory cutaneous lupus erythematosus.
What are the precautions and contraindications to rituximab?
Precautions
- The use of rituximab in children and patients with kidney or liver failure has not been studied extensively.
- Rituximab should be avoided during pregnancy unless the potential benefit justifies the potential risk to the fetus (Pregnancy Risk Category C).
- Breastfeeding mothers should be advised to discontinue nursing until circulating blood levels are no longer detectable.
- Caution with use in those with HIV infection and a CD4 cell count <250/microlitre.
Current safety information is based on treatment for non-Hodgkin lymphoma and rheumatoid arthritis and may not be applicable when rituximab is used for other disorders.
Drug interactions
Though there have been no formal drug interaction studies with rituximab, concomitant use with cisplatin should be avoided as this combination has been associated with renal failure.
Contraindications
- Hypersensitivity to rituximab or other murine proteins.
- Severe active infections.
- Severe heart failure.
Pre-treatment considerations and monitoring on rituximab
Prior to treatment
- Check immunoglobulin levels as well as routine haematology and biochemistry.
- Low IgG levels are associated with a higher risk of serious infections induced by rituximab.
- Screen for and treat infections such as hepatitis B.
- Pre-treatment immunisation should be considered especially pneumococcus, influenza, and hepatitis B.
Monitoring
- The blood count should be monitored monthly, then every three months, as neutropaenia may occur.
Dosage and administration of rituximab
Prior to the rituximab infusion, premedication is administered 30 minutes in advance. This usually includes paracetamol and an antihistamine and may include glucocorticoids.
Determining the optimal dosage and regimen of rituximab for pemphigus treatment remains a topic of ongoing discussion. Various therapy approaches are currently employed, including the following protocols.
- Lymphoma protocol — the most widely utilised regimen involves administering rituximab once a week for 4 weeks at a dose of 375 mg/m2 body surface area. Multiple studies have supported this protocol.
- Rheumatoid arthritis protocol — in this approach, rituximab is given twice, with a 15-day interval. Dermatologists have increasingly adopted this protocol for managing pemphigus, with advantages such as reduced cost and fewer infusion sessions compared to the lymphoma program.
- Combination therapy — rituximab has been combined with other treatments, including IVIG (intravenous immunoglobulin), immunoadsorption, and dexamethasone pulse therapy, among others.
- Maintenance therapy — following an initial induction cycle, rituximab infusions may be administered every 4 or 12 weeks throughout the treatment duration.
Rituximab is given by IV infusion. The total infusion duration is approximately 5 to 6 hours, depending on the infusion rate.
What are the side effects and risks of rituximab?
Most patients experience mild-to-moderate side effects from their first infusion of rituximab. The most common symptoms are:
- Fever (48%)
- Chills (32%)
- Weakness (18%)
- Nausea (17%)
- Headache (13%)
- Itch (12%)
- Rash (11%).
The symptoms usually settle if the infusion is stopped temporarily and tend to be less severe with subsequent infusions. Pre-treatment with paracetamol and antihistamines is recommended. A corticosteroid may also be used.
Although infections are rare, some patients have died after rituximab infusions due to:
- Bacterial sepsis
- Reactivation of hepatitis B with fulminant hepatitis
- Progressive multifocal leukoencephalopathy — a viral infection affecting the central nervous system.
Patients should be urged to seek prompt medical attention if they develop new neurological symptoms such as changes in vision, balance, or thought processes.
Other deaths from rituximab have been due to:
- Tumour lysis syndrome
- Infusion reactions
- Stevens-Johnson syndrome / toxic epidermal necrolysis.
Many other less severe adverse reactions have been reported, including a variety of rashes and:
- Stomatitis
- Alopecia (hair loss)
- Urticaria
- Flushing
- Hyperhidrosis.
We suggest you refer to your national drug approval agency such as the Australian Therapeutic Goods Administration (TGA), US Food and Drug Administration (FDA), UK Medicines and Healthcare products regulatory agency (MHRA) / emc, and NZ Medsafe, or a national or state-approved formulary eg, the New Zealand Formulary (NZF) and New Zealand Formulary for Children (NZFC) and the British National Formulary (BNF) and British National Formulary for Children (BNFC).