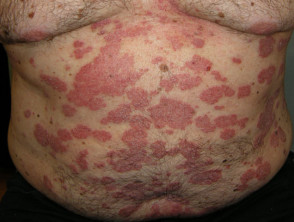
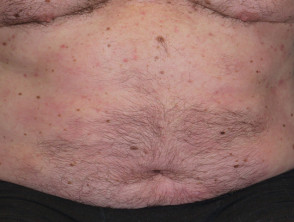
What is psoriasis?
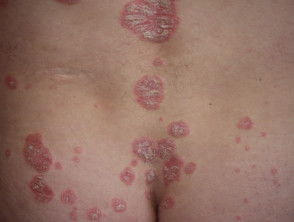
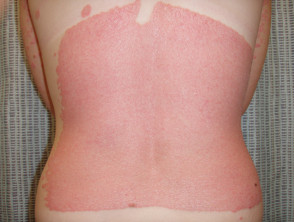
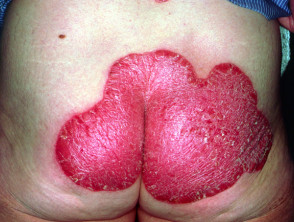
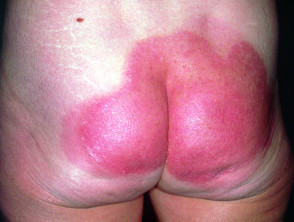
Psoriasis is a common, chronic, immune-mediated skin disease with characteristic red, scaly plaques caused by the excessive proliferation of skin cells.
There are a number of types of psoriasis, including chronic plaque psoriasis, guttate psoriasis, flexural psoriasis, palmoplantar psoriasis, and nail psoriasis.
Psoriasis
Principles of treatment
Despite recent advances in our understanding of the mechanism of how psoriasis develops, psoriasis may be difficult to treat; there is currently no cure and no single treatment works for everyone.
Several treatments may need to be tried before the most suitable regime is established, and different treatments may need to be used concurrently, or in rotation, for best effect or to minimise side-effects.
Treatment of adults with psoriasis includes:
- General measures
- Topical preparations
- Ultraviolet therapy
- Systemic non-biological therapy
- Systemic biological therapy.
Treatment choice in psoriasis depends on a number of factors. For example:
- Disease pattern
- Disease severity: body surface area (BSA) affected and Psoriasis Area and Severity Index (PASI) score
- Disease impact: symptoms and Dermatology Life Quality Index (DLQI) score (see Psychological effects of psoriasis)
- Patient preference
- Acceptability and practicalities of treatment
- Patient age and general health
- Comorbidities (eg, psoriatic arthritis, kidney or liver disease [see Liver problems and psoriasis])
- Other medications
- Conception plans or current pregnancy
- Treatment goals (eg, improving nail psoriasis or aiming for a 90% improvement in PASI score [PASI 90])
More details about each form of psoriasis treatment are found on individual topic pages.
General measures used to treat psoriasis
Avoidance of triggers
Where possible, minimise factors that aggravate or trigger psoriasis, such as stress, streptococcal infections, and certain medications (lithium, beta-blockers, and antimalarial drugs). [see Drug-induced psoriasis]
Treatment of associated conditions
Health conditions associated with psoriasis include psoriatic arthritis, sleep disturbance, and depression. Treatment for these may help skin disease.
Due to the association between psoriasis and metabolic syndrome, weight loss, smoking cessation, moderation of alcohol intake, and blood pressure control may also lead to improvements in skin disease [1,2].
Sun exposure
Sun exposure (heliotherapy) may help to clear psoriasis; in many people, psoriasis improves dramatically during summer months or on sunny holidays.
- Short-term side-effects include the development of psoriasis in areas of sunburn, (due to the Koebner phenomenon).
- Long-term risks include premature ageing of the skin and the development of skin cancer.
Psoriasis aggravated by sun exposure
Baths
Soaking in warm water can soften the psoriatic plaques and lift the scale.
- Soap substitutes or bath oils are useful.
- Antiseptics are not necessary and may cause skin irritation.
- Balneotherapy (the treatment of disease by bathing in mineral springs) is a popular form of complementary therapy in certain demographic areas [3], although there is little or no strong evidence of benefit.
Probiotics
One avenue of current research is looking at the skin and gut microbiome (the bacteria living on and in the human body) and whether the alteration of this microbiome may be effective in the treatment of psoriasis [4,5]. To date, probiotics have not been found to help psoriasis.
Occlusive dressings
Relatively small, localised patches of psoriasis may improve with occlusion (eg, using waterproof adhesive dressings).
Topical preparations for psoriasis
Emollients
The regular use of emollients and moisturisers softens psoriasis and adds moisture to the skin. This improves dryness, scaling, and irritation.
- There are a diverse range of options of lotions (ie, for scalp psoriasis), creams, and ointments (ie, for dry, thick, scaly areas).
- Thick ointments based on white soft paraffin are often recommended for chronic plaques and hand or foot psoriasis.
- They should be applied liberally and frequently.
Be aware of the flammability of emollients and the risk of slipping in the bath after applying these agents to the feet. Emollients can rarely irritate the skin; this is less likely with ointments than with lotions and creams.
Keratolytic agents
Keratolytic agents can be useful to reduce the thick scale. They may contain urea (5–40%), salicylic acid (0.5–10%) or propylene glycol (for example, propylene glycol 20% in aqueous cream).
Topical steroids
Topical steroids are safe and relatively easy to use for plaque psoriasis, scalp psoriasis, flexural psoriasis, sebopsoriasis, and psoriasis affecting the palms and soles. They are not very effective in nail psoriasis.
Topical steroids are available in various strengths and formulations (including steroid impregnated tape). Topical steroids are also used in combination with other agents, such as with:
- Calcipotriol (a vitamin D-like compound)
- Salicylic acid (a de-scaling agent)
- Antifungal agents for flexural psoriasis (to counteract Candida albicans).
The selection of a suitable product depends on the site and type of psoriasis.
- Weak topical steroids are used on sensitive sites (ie, the face, flexures, and genital areas).
- In contrast, palmoplantar psoriasis requires a very potent topical steroid due to the thicker skin on the hands and feet.
Potent steroids are often more effective than mild topical steroids, but they have a higher risk of side-effects. They should be used with caution in large areas and for limited periods. They may cause:
- Skin atrophy and/or striae
- Telangiectasia and/or purpura
- Aggravation of plaque psoriasis
- Triggering of an episode of pustular psoriasis.
Topical steroids are useful for the itch of psoriasis, and work well for the inflammation initially. However with continued use tachyphylaxis develops, and the anti-inflammatory effect wears off.
Topical steroids can be used under medical supervision in pregnancy and, alongside emollients, are generally the first-line treatment of psoriasis in pregnancy.
Intralesional steroid injections may be used for a small number of thickened plaques of psoriasis and in nail psoriasis [6].
Vitamin D-like compounds
Vitamin D-like compounds for psoriasis include calcipotriol, calcitriol, and tacalcitol.
- They are applied once or twice daily.
- They reduce the thickness and scaliness of plaques.
- Redness may persist.
Calcipotriol may be used for chronic plaque psoriasis and scalp psoriasis, whereas calcitriol ointment is often preferred for flexural psoriasis or genital psoriasis (as it is less irritating than calcipotriol).
- If irritation occurs, reduce the frequency of application to every second day or less often for a period of time.
- Vitamin D-like compounds may cause a facial rash, so these treatments are not usually suitable for facial psoriasis.
- No more than 100 g should be used each week.
When combined with ultraviolet (UV) therapy, calcipotriol should be applied after to exposure to UV radiation, because:
- UV radiation deactivates calcipotriol.
- Calcipotriol acts as a sunscreen.
Vitamin D-like compounds are best avoided in children under 6 years, and during pregnancy and lactation, due to lack of data regarding their safety.
Calcipotriol is available in combination with a very potent topical steroid, betamethasone dipropionate as a gel/ointment or foam.
- This is often the first line of treatment in plaque psoriasis.
- It should not be used continuously or over large areas due to the risks associated with excessive steroid use.
Effect of calcipotriol ointment on chronic plaque psoriasis
Tar
Coal tar may be applied as solutions, lotions, creams, ointments, gels, and shampoos.
- It is often mixed with other ingredients.
- It is particularly effective for scalp psoriasis and large thin plaque psoriasis.
Care needs to be taken following the application of coal tar treatments.
- They can irritate the skin, particularly on initial use.
- They can be messy (ie, staining skin, hair, and clothing), and often have an associated odour.
- Sunlight can interact with tar on the skin to cause a sunburn-like photocontact dermatitis.
Dithranol
Dithranol (also called anthralian or anthralin) is occasionally recommended as a treatment for chronic plaque psoriasis. It can be very effective but dithranol treatment has a number of practical drawbacks and so is less frequently prescribed.
The method of application is complex; it is usually given as ‘short contact' therapy.
- Dithranol is normally applied once a day.
- It is applied directly to psoriasis (ie, avoiding normal skin) and then washed off after 10–60 minutes.
- The strength of dithranol is gradually increased every few days until it is effective, or until skin irritation occurs.
- Dithranol permanently stains fabrics and temporarily stains the skin.
Calcineurin inhibitors
The calcineurin inhibitors are tacrolimus ointment and pimecrolimus cream.
- They are mostly used to treat atopic dermatitis, and their use for psoriasis is off-licence.
- They are used as steroid-sparing agents on sensitive sites where the skin is thinner (eg, the face, flexures, and genital areas).
- They are not effective for treating chronic plaque psoriasis elsewhere (unless under occlusion) [7].
Tazarotene
Tazarotene is a topical retinoid, which may be applied once daily to plaque psoriasis as a 0.05% or 0.1% cream. [8].
The most common side-effect is skin pain and local irritation. It is not currently available in New Zealand (July 2018).
Roflumilast
Topical roflumilast 0.3% cream (Zorvye™) was approved by the FDA in 2022 for the treatment of plaque psoriasis in patients aged 12 years and older. This is the first topical PDE4 inhibitor approved for use in psoriasis.
Tapinarof
Tapinarof 1% cream (VTAMA®) is a once-daily, novel, topical agent for patients with mild, moderate, and severe plaque psoriasis, first approved by the US Food and Drug Administration (FDA) in May 2022.
Other
Off-label use of the phosphodiesterase-4 topical inhibitor crisaborole has been shown to be effective for flexural and facial psoriasis.
Ultraviolet treatment for psoriasis
Phototherapy is the use of UV radiation to treat skin disorders, and this can be very effective in the treatment of psoriasis. It is generally reserved for cases where topical therapy has been ineffective or too much of the skin surface is involved to treat psoriasis effectively with topical agents. It is administered in cabinets at specialised centres, and a treatment course for psoriasis will usually consist of 2–3 treatments per week for 20–30 treatments.
- Phototherapy is best avoided in patients with very fair skin, who take certain immunosuppressive medications, or who have a previous history of skin cancer.
- It is not effective for flexural sites.
- Early side-effects include sunburn and photosensitivity rashes.
- Late side-effects include ageing of the skin and skin cancer.
The need for regular travel to a phototherapy centre can make this option difficult for some patients. The beneficial effects may be short-lived.
Narrow ultraviolet B
Narrowband ultraviolet B (UVB) (311–312 nm wavelengths) is also known as TL01 light therapy (after the type of fluorescent tubes used).
- Narrowband UVB is particularly effective in thin chronic plaque psoriasis and guttate psoriasis, especially in the winter months.
- It is generally felt to be safe and well-tolerated. About two-thirds of patients with plaque psoriasis experience a 75% improvement in PASI score (PASI 75) compared to baseline with this treatment [9].
- UVB is felt to be safe in pregnancy. Note that UVB degrades folic acid and regular supplementation in pregnancy is needed [10,11].
Psoralen and ultraviolet A
Psoralens plus long wave ultraviolet A (UVA) radiation, (known as photochemotherapy), can be applied to the whole body by giving an oral psoralen in tablet form 2 hours prior to treatment.
- Treatment can be localised to the hands and/or feet by using psoralen bath soaks or topical psoralens prior to treatment.
- Localised treatment is commonly used to treat thick plaques or moderate-to-severe hand and/or foot psoriasis, including palmoplantar pustulosis.
- Photosensitivity persists for some hours following oral psoralen treatment; therefore, patients are advised to avoid sun exposure, including wearing wrap-around sunglasses on the day of treatment.
- PUVA is more likely than narrowband UVB treatment to cause skin cancer, especially squamous cell carcinoma and is usually limited to a maximum of 100 to 200-lifetime treatments.
- Psoralens and therefore PUVA is not recommended during pregnancy or breastfeeding.
Systemic non-biological medication for psoriasis
Systemic (oral or injectable) medication may be required to treat psoriasis when:
- Topical therapy is ineffective
- Psoriasis is affecting the patient's physical, social, or psychological health
- Psoriasis is severe
- Phototherapy is ineffective or contraindicated.
The selection of the appropriate medication is specific to each individual patient, as each agent carries its own risks and benefits.
As a rule, systemic steroids should be avoided as they can make psoriasis worse or unstable and difficult to control with other treatments.
Methotrexate
Methotrexate is an immunomodulatory and anti-inflammatory treatment used to treat psoriasis, psoriatic arthritis, and Crohn disease.
- Methotrexate is usually given once a week.
- Oral tablets or subcutaneous injections may be selected.
- Folic acid supplementation is often added.
The dose of methotrexate is often adjusted over the first few weeks or months of treatment. There are various regimens.
- It can be given long-term if there are no significant side-effects.
- 45% of patients treated with methotrexate for 12–16 weeks experience PASI 75 [13].
- Regular blood tests should monitor liver function and blood count while the patient is on methotrexate.
- Excess alcohol should be avoided.
- Methotrexate can cause fetal harm so women should not become pregnant while taking methotrexate and for 3 months after stopping it.
Side-effects of methotrexate include nausea, tiredness, mouth ulcers, and diarrhoea.
Ciclosporin
Ciclosporin is an immunosuppressant used short-term to treat atopic eczema and psoriasis.
- Ciclosporin capsules are usually taken twice daily.
- The dose is individualised according to patient weight, efficacy, and adverse effects (2.5–5.0 mg/kg/day).
Ciclosporin has a rapid onset of action, making it useful in severe plaque psoriasis or pustular psoriasis.
- Due to its side effects and risks, ciclosporin is usually prescribed in courses of 8–12 weeks.
- 50–80% of patients treated with ciclosporin for 8–12 weeks achieve PASI 75 [14].
- Regular blood tests should be undertaken and monitor at least the patient's blood pressure and renal function.
- There is no evidence that ciclosporin causes fetal harm but doses should be kept low in pregnancy or it should be discontinued due to hypertension.
- It must not be taken when breastfeeding.
Important side-effects of ciclosporin include hypertension, renal failure, susceptibility to infection, and increased risk of skin cancer.
Acitretin
Acitretin is a vitamin A-like compound or retinoid that is particularly effective for palmoplantar psoriasis.
- Acitretin capsules are usually taken once daily.
- Dose varies from 10 mg three times weekly to 50 mg daily.
- Acitretin is often combined with phototherapy
- 20–40% of patients treated with acitretin at full dose achieve PASI 75 by Week 16 [14].
- Regular blood tests should monitor the patient's liver function and blood fats.
- Pregnancy must be strictly avoided while on acitretin and for at least 3 years afterwards because it is associated with serious birth deformities (the US and TGA pregnancy category X). Acitretin is therefore rarely given to women of childbearing age.
- The risk does not apply to men, as acitretin does not affect sperm.
Dose-related side-effects of acitretin include dry lips, peeling palms and soles, thinning hair, tiredness, and muscle pains.
Apremilast
Apremilast is a phosphodiesterase-4 inhibitor used to treat psoriasis and psoriatic arthritis. It is not funded in New Zealand (July 2018).
- The dosing of apremilast is titrated for the first 5 days and then given as a fixed-dose twice daily.
- Treatment may be given long-term and does not require drug screening or monitoring.
- 29–33% of patients treated with apremilast achieve PASI 75 at week 16 [14].
- Apremilast is thought to be harmful to a developing fetus and so it should not be given in pregnancy (US pregnancy category C).
Side effects of apremilast include nausea and diarrhoea. There have also been reports of suicidal behaviour.
Fumaric acid esters
Fumaric acid esters are immunosuppressants. They are not available or funded in New Zealand.
- The dose of fumaric acid is slowly escalated.
- Several different preparations are available.
- The dose is usually gradually increased to achieve an effect. PASI 75 is achieved 38% of patients at Week 16.
- Blood tests should monitor the patient's kidney, liver, and white blood cells.
Side-effects include nausea, diarrhoea, stomach cramps, flushing, and headaches. A rare but serious side-effect is a nervous system viral infection (progressive multifocal leukoencephalopathy).
Fumaric acid esters are not to be used during pregnancy due to fetal harm.
Other non-biological medications
Other non-biological oral medications used less commonly for psoriasis include:
Biological therapies
Biological therapies or biologics are monoclonal antibodies or recombinant proteins targeted at specific components of the immune system. They are often very effective treatments for psoriasis.
- The risk of serious side-effects relates to their effect on immunity.
- They are very expensive and the prescription is tightly regulated.
- They are used for moderate-to-severe psoriasis that has failed on topical and systemic treatment options or when these are contraindicated.
Each available biologic has individual risks and benefits. Novel biological therapies are under development. Currently available biologics and those under development include:
- Tumour necrosis factor-alpha (TNFα) inhibitors: infliximab, etanercept, adalimumab, certolizumab, and golimumab
- Interleukin 17 (IL-17) agents: secukinumub, ixekizumab, and brodalumab
- Interleukin 12 and 23 (IL-12 and IL-23): ustekinumab, tildrakizumab, guselkumab, and risankizumab.
The biologicals for psoriasis are given by subcutaneous injection, with the exception of infliximab, which is administered intravenously. Infliximab is a chimera of mouse and human protein and can result in infusion reactions and antibody formation.
Biosimilars are drugs that are nearly identical to an original biological medication that has come off patent [15] and are available at a reduced cost. Biosimilars are available for infliximab and etanercept and others are under development (July 2018).
Oral drugs under development include small molecule compounds targeting signalling pathways, such as JAK inhibitors, PDE inhibitors. As they are low molecular weight, topical formulations are being investigated.
Effect of adalimumab on psoriasis